Abstract
In prostate cancer (PC), increasing evidence suggests that androgen receptor (AR) signalling is functional under conditions of maximal androgen blockade. PC cells survive and proliferate in the altered hormonal environment possibly by interactions between growth factor-activated pathways and AR signalling. The present review article summarizes the current evidence of this crosstalk and focuses on the interactions among the ErbB receptor network, its downstream pathways, and the AR. The potential role of this crosstalk in the development of androgen independence and in relation to antiandrogen therapy is discussed. Such interactions provide insight into possible complementary or additional strategies in the management of PC.
Keywords: androgen receptor, ErbB receptors, MAPK, prostate cancer, AKT
Introduction
Prostate cancer (PC) is the commonest cancer affecting men in the western world. The number of new cases of PC registered in England and Wales (age-standardized) increased by 104% from 1971 and 1993, whereas the number of deaths increased by 38% between 1971 and 1998 [1]. In the year 2000, there were an estimated 180,400 new cases and 31,900 deaths in the United States [2]. Conventional therapies involve surgical or pharmacological castration [clinically referred to as maximum androgen blockade (MAB) when combined with antiandrogen therapy] with the intent of maximally diminishing the availability and action of androgen on the androgen receptor (AR). Over time, the PC cells overcome the need for androgen as a survival, growth, and differentiating factor and become androgen-independent (AI) [3,4]. Evidence from xenograft models and clinical material indicates that this change to AI growth could result from either the outgrowth of a preexisting (pretreatment) AI clone or adaptive responses to androgen deprivation, which allow the evolution of AI clones [5,6]. Under conditions of MAB, autocrine growth factor loops could enhance growth and mitogenic signalling through the activation of tyrosine kinase-coupled receptors and subsequently second messengers such as mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), and other effector pathways.
In the last decade, it has become clear that signalling pathways are not simply linear sequences of interactions downstream of an activated receptor. Growth factors appear not to act independently of the AR in the prostate. Crosstalk between these signal transduction pathways has been implicated in maintaining PC survival in an androgenpoor environment [7]. In this review, evidence demonstrating the potentially important crosstalk mechanisms that bypass classical androgen dependence is presented.
AR
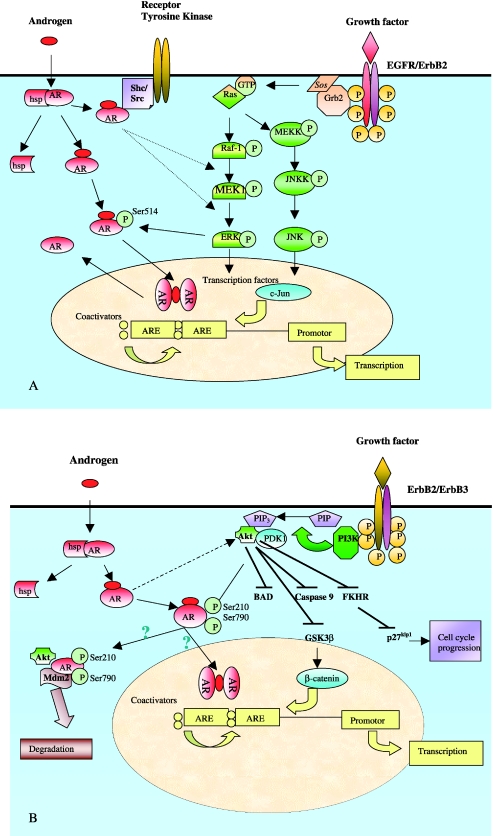
The AR is a member of the nuclear receptor superfamily of transcription factors [8]. It can be localized to the cytoplasm or nucleus and its topographic localization is a reflection of its functional state (active/inactive). In its inactive state, AR associates with heat shock proteins (hsp70 and hsp90) [9,10]. In the absence of ligand, cytoplasmic AR is degraded. In the presence of the ligand, testosterone, and its more potent derivative dihydrotestosterone (DHT), the AR-hsp complex is disrupted and the AR undergoes conformational change that allows phosphorylation, dimerization, and translocation of the more stable ligand-receptor complex to the nucleus [11,12]. In the nucleus, the dimerized AR-ligand complex interacts with coactivator molecules (e.g., ARA54, ARA70) [13] and initiates gene transcription of androgen-regulated genes by binding to specific androgen response elements (AREs). (The different states of the AR and its localization are illustrated in Figure 1, A and B.) The gene activation pathways initiated by androgens impact upon the processes of prostatic cellular proliferation, survival, and differentiation [14].
Figure 1.
(A) The interaction between the AR and the ErbB/MAPK pathway. Parallel pathways activated by androgen and the ErbB receptor intersect at several levels. Following hormone binding in the cytoplasm, the AR-hsp-containing complex is dissociated and the steroid receptor is rapidly translocated to the nucleus. In the nucleus, AR dimerizes and binds to the DNA double helix at specific sequences called AREs. The DNA-bound AR dimer recruits a multiprotein complex containing members of the basal transcription machinery and other coactivators or corepressors (not shown), which control the transcriptional AR response. AR is then dissociated from the DNA and shuttled back to the cytoplasm where it can reassociate with hsp or ligand. Growth factors initiate signalling by binding to and sequentially activating the ErbB receptors, adaptor molecules like Grb2 and Sos, and the GTP exchange factor Ras. This in turn activates the three-tiered MAPK cascade. Crosstalk may occur in the cytoplasm, for example, when AR complexes with Shc/Src (two adaptor molecules recruited by activated receptor tyrosine kinases) to activate the MAPK pathway, leading to phosphorylation of ERK. Alternatively, ErbB2 activates the AR through ERK phosphorylation at Ser514, thereby suggesting an influence of these two pathways on each other. c-Jun is activated downstream of MEKK, which enhances the homodimerization of AR with DNA and consequently the AR response to androgen. The requirement for AR in MEKK1-induced apoptosis is not shown in this figure. (B) The interaction between AR and ErbB/PI3K/Akt. Similar to (A), ligand binding of the AR initiates dissociation from hsp, phosphorylation, and dimerization. Growth factors activate ErbB2/ErbB3 complex and recruit PI3K, which produces 3′-phosphoinositides (PIP3) and recruits PDK1 and Akt, resulting in their activation. Akt then inactivates a variety of proapoptotic molecules including Bad, caspase 3, GSK-3β, and the forkhead transcription factors (FKHR). In addition, Akt targets the AR and phosphorylates it at Ser210 and Ser790. This phosphorylation promotes PC cell survival by controversial mechanisms (marked by the question marks). Some workers hypothesize that this is through activation of AR transcription. Alternatively, it may protect the cells from androgen-induced apoptosis through ubiquitylation and degradation of the AR within an Akt/Mdm2/AR complex. Another mechanism of AR activation by Akt is through GSK-3β inactivation and nuclear accumulation of β-catenin. In the cytoplasm, AR rapidly activates PI3K and increases intracellular PIP3 by an as yet unknown mechanism. PTEN, the negative regulator of this pathway, is not shown in this figure.
AI growth may be attributed to secondary genetic mutations, which allow the AI PC cells to survive and proliferate despite the paucity/absence of androgen and imply that signalling through the AR is no longer active [15–17]. However, in nearly all cases of AI PC, the persistent expression of AR and of androgen-regulated genes such as PSA indicates that AR signalling is not always bypassed but is indeed functional and active [7].
Augmentation of AR-mediated signal at lower androgen levels can occur by amplification of the AR gene itself. AR gene amplification was observed in 30% of recurrent PC tumor specimen after androgen deprivation therapy [18–23]. AR hypersensitivity and increased AR protein stability have also been implicated in AI PC progression [24]. Several in vitro studies on human PC cell lines have shown that mutations in the AR can make the receptor “promiscuous”—whereby the AR can be activated by a number of different ligands such as testosterone, DHT, estrogen, progesterones, and the adrenal androgen dihydroepiandrosterone (DHEA) [25]. To complicate matters further, antiandrogens such as flutamide, hydroxyflutamide, and bicalutamide can also activate mutant AR [26]. These studies suggest that AR mutations in PC may provide a survival advantage for AR-mutated clones in response to treatment with antiandrogens as flutamide [27,28]. In clinical PC, AR mutations have been observed [29,30] and are thought to be the most likely explanation for the “antiandrogen withdrawal syndrome.” This syndrome is a well-established phenomenon in PC where a subset of patients will benefit from withdrawal of antiandrogen hormonal therapy and exhibit decreasing PSA values and clinical improvement [31]. Other researchers have reported that antiandrogens, hydroxyflutamide, bicalutamide, cyproterone acetate, RU58841, and other compounds such as genistein and RU486, can promote the interaction between AR and its coactivator, ARA70, in a dose-dependent manner [32]. Increased expression or mutations in AR coactivators can activate the AR in the absence of DHT [e.g., cofactor ARA70 specifically conferred the androgenic effect from 17 β-estradiol (E2) and hydroxyflutamide to AR] [33,34].
An alternative model of AR activation in the setting of MAB involves nonsteroid receptor signal transduction pathways [35]. AR can be activated in a ligand-independent manner by a number of growth factors including insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF), keratinocyte growth factor (KGF), and interleukin-6 (IL-6). Each of these has been shown to transcriptionally activate an androgen-responsive reporter construct in the absence of ligand or synergistically in conjunction with androgens. The mechanistic details are, however, unknown [36–38]. The effect exerted by growth factors on the AR suggests that these signalling pathways are not mutually exclusive. They point towards the existence of crosscommunication between ligands, at the level of their cognate receptors or through their intracellular downstream kinase cascades, and the AR. This allows the emergence of AI cells, which can survive and proliferate under androgen ablation, leading to recurrence and metastasis [7,37].
Data on the importance of the interaction between polypeptide growth factors like EGF and the ErbB network of receptors (or their secondary mediators) with the AR in favor of PC survival are now rapidly emerging. This article reviews these interactions and focuses on their potential role in promoting AI growth.
AR and the ErbB Receptors
ErbB receptors are typical receptor tyrosine kinases activated downstream of EGF and EGF-like ligands. They include four members: EGF receptor EGFR/ErbB1, ErbB2 (or HER2/Neu), HER3/ErbB3, and HER4/ErbB4. All EGFR family members are characterized by a modular structure consisting of an extracellular ligand binding domain, a transmembrane region, and an intracellular part harboring the highly conserved tyrosine kinase domain. Ligand binding induces the formation of homodimers or heterodimers and the phosphorylation of tyrosine residues, which serve as docking sites for a variety of signal transducers (Figure 1, A and B) [39,40]. This family of receptors plays a critical role in the proliferation, migration, survival, and differentiation of target cells. Dysregulation of signalling by the ErbBs has been implicated in the pathogenesis and progression of human cancers [39,40].
ErbB1/EGFR
EGF is a mitogen required for normal prostatic epithelial cell growth and is present in large amounts in human prostatic fluid [41,42]. mRNA and protein expression of its receptor, EGFR, have been demonstrated in human PC, benign prostatic hyperplasia (BPH), and normal prostate [43,44]. Transforming growth factor-α (TGF-α) is another ligand for EGFR. The existence of a stimulatory autocrine loop involving EGF, TGF-α, and EGFR has been suggested from in vivo observations in high-grade prostatic intraepithelial neoplasia (PIN) and PC. These lesions showed a higher expression of membranous EGFR, ErbB2, and cytoplasmic TGF-α than low-grade PIN or BPH [45]. This stimulatory loop appeared to be important in the growth of three human PC cell lines: PC-3, LNCaP, and DU145. The DU145 cell line was apparently dependent on this autocrine loop for cell proliferation [46]. The importance of the EGFR in promoting the survival of PC cells was demonstrated in the CWR22 xenograft model of PC, in which castration was associated with an initial reduction in EGFR expression. EGFR returned to comparable or higher levels following the administration of androgen or in relapsed AI tumors (CWR22R) [47].
DHT and EGF are thought to play complementary roles in regulating the proliferation of prostate cells. The combination of DHT and EGF has been shown to enhance the proliferation of the MDA PC2a and MDA PC2b human PC cell lines through a convergent stimulatory mechanism. This promoted progression through the cell cycle by increasing cyclin-dependent kinase-2 (CDK2) activity and accelerating the downregulation of the CDK inhibitor p27kip1 compared to either ligand alone [48]. In addition, an interactive stimulation of EGFR synthesis by DHT [47–51] and of AR synthesis by EGF [48] has been described in several androgen-sensitive PC cell lines. DHT increased both EGFR expression and receptor-ligand affinity, resulting in increased EGF binding and an enhanced mitogenic response to EGF [47–51]. This effect of DHT can be blocked by the AR antagonist bicalutamide, which indicates that these effects require the AR [47].
ErbB2 (HER2/Neu)
ErbB2 is one of the best-studied genes involved in human malignancy. In PC, ErbB2 is considered a potential surrogate biomarker for screening chemopreventive agents in short-term Phase II trials [52,53]. Unlike other members of the ErbB family, ErbB2 has no known ligand and is the preferred heterodimerization partner within the EGFR family. Heterodimers containing ErbB2 induce signals with the strongest biological activity [39]. EGFR and ErbB2 serve as receptors for EGF [40] and so they are likely to be involved in an interaction between the EGF and AR signalling pathways.
Studies of the role of ErbB2 in PC remain inconclusive. Previous work has shown widely divergent levels of ErbB2 expression in primary PC, probably owing to methodological differences in the studies. Some authors have demonstrated ErbB2 protein overexpression and/or gene amplification in a subset of PC patients and in premalignant lesions [54–58]. ErbB2 protein was expressed at statistically significant higher levels in PC treated by androgen deprivation therapy compared to untreated cancer [57,59]. On the other hand, some investigators have not found overexpression of ErbB2 in PCs [60] and most have not shown amplification of the ErbB2 gene [59,61,62]. Several authors have suggested that elevated serum levels of the extracellular portion of ErbB2 in patients with metastatic PC provide further supporting evidence of its potential role [63,64]. Using the LAPC-4 mouse xenograft model, Craft et al. [65] showed that AI sublines of human PC xenografts expressed higher levels of ErbB2 than androgen-dependent sublines. They also showed that overexpression of ErbB2 confers AI growth to the androgen-dependent LNCaP cell line. In the absence of androgen, overexpression of ErbB2 activated the transcription of prostate specific antigen PSA (a process that requires a functional AR but is not inhibited by the antiandrogen bicalutamide) [65]. The failure of bicalutamide to block PSA induction by ErbB2 is consistent with clinical AI PC and indicates that ErbB2 interacts with the AR pathway distal to the interaction between androgens and AR.
The biochemical mechanisms of this crosstalk are unclear, but the failure of ErbB2 to activate a single highaffinity AR binding site points either to the involvement of an intermediate protein, or that ErbB2 may optimize AR function by activating Ras and other signalling pathways (Figure 1A). Ras proteins are molecular switches with the ability to interact and activate several effector molecules. Among those, Raf-1 kinase, PI3K, and Ral-GDS are the best characterized. Raf activates the mitogenic MEK/ERK kinases pathway, whereas PI3K regulates the PKB/Akt cascade, involved in the control of proliferation, metabolism, and apoptotic responses (both pathways are discussed in detail below and outlined in Figure 1, A and B, respectively). Yeh et al. [66] demonstrated ErbB2 activation of the AR through activating the MAPK pathway as well as promoting the interaction of AR and ARA70 coactivator. ErbB2 overexpression thereby favors AR activation at very low levels of androgen.
ErbB2 has also been involved in signalling downstream of other ligands such as IL-6. As previously mentioned, IL-6 is a nonsteroidal activator of the AR whose level is frequently elevated in sera of patients with metastatic PC [67]. Through a novel mechanism that involves the heterodimerization of the IL-6 receptor and ErbB2, IL-6 activated both ErbB3 and MAPK in LNCaP cells [68].
ErbB3/HER3 and ErbB4/HER4
Neu differentiation factor/heregulin (HRG) belongs to a family of polypeptide growth factors that bind to receptor tyrosine kinases ErbB3 and ErbB4. HRG binding induces ErbB3 and ErbB4 heterodimerization, activating downstream signal transduction. ErbB3 differs from other ErbB family members in that it possesses diminished kinase activity and is largely dependent upon other ErbB kinases, in particular ErbB2 [39,69].
HRG is present in normal human adult prostate and BPH, where it may function as a paracrine physiological differentiation factor [70,71]. Analysis of clinical PC specimens indicates that overexpression of ErbB3 has been linked to a less favorable prognosis [72]. In PC cell lines, HRG inhibited the growth of AR-positive but not AR-deficient cells [71]. The exact sites of crosstalk between HRG and AR signalling pathways remain to be elucidated. However, the fact that only AR-positive PC cells appear to be influenced by HRG supports the concept that the physiological function of HRG on prostatic cells is optimal in the presence of AR.
Ebp1 is a recently identified ErbB-3 binding protein that possesses an important motif, which mediates interactions with nuclear hormone receptors and is thought to provide a link between the ErbBs and AR. Ebp1 has been shown to bind to AR in vitro and in vivo. It inhibited ligand-mediated transcriptional activation of AR-regulated genes such as the PSA growth of AR-positive LNCaP cells [73].
Reports on the ErbB4 in the prostate are few. ErbB4 protein is strongly expressed by normal prostate luminal cells. Only 23% of PC specimens and none of the PC cell lines examined so far expressed ErbB4 [71]. More investigations into the role of ErbB4 in the prostate are required.
Interactions between the AR and the ErbB receptors are summarized in Table 1.
Table 1.
A Summary of the Major Interactions between the AR and ErbB-Mediated Signalling.
| Interaction with the AR | Effect on cell | |
| EGF/EGFR | EGF augments DHT and AR signalling, and stimulates AR synthesis [48] | Enhanced proliferation and cell cycle progression |
| DHT stimulates EGFR synthesis [47–51] | Enhanced mitogenic response to EGF | |
| ErbB2 | Transcriptional activation of PSA [65], enhancement of AR coactivator binding [66], activation of MAPK signalling downstream of IL-6 [68] | Ligand-independent activation of AR, which promotes survival and proliferation of PC in the androgen-depleted environment |
| HRG/ErbB3 | HRG acts on AR positive cells only [71] possibly through ErbB-3 binding protein Ebp1, which binds to AR in vitro and in vivo [73] | Inhibition of ligand-mediated transcriptional activation of AR and reduced growth of AR-positive cells |
| MAPK/ERK2 | Activation of AR transcription downstream of ErbB2 and IL-6 [66], phosphorylation of the AR at Ser514 [66] and SRC-1 [93] | Optimal ligand-dependent and ligand-independent activation of the AR |
| AR activates MAPK in a complex with Src/Shc [90,91]. | A rapid nongenomic effect that cannot be inhibited by antiandrogens [89] | |
| MEKK1 | Transcriptional activation of AR [94] possibly through its downstream target c-Jun, which interacts with the DNA binding domain/hinge region of AR [96,97] | Potentiation of the functional interaction between N-terminus and C-terminus of AR to enhance DNA binding and gene transcription |
| Activates apoptotic pathways only in AR-expressing cells [94] | Apoptosis of PC cells | |
| Akt | Phosphorylates AR at Ser210 and Ser790, which either activates transcription of AR-regulated genes in the absence of androgens [109,114]; alternatively, phosphorylation and inactivation of GSK-3β increased nuclear levels of β-catenin, which elevates AR activity [118] | Increased survival of PC cells in the absence of androgen |
| Repression of AR transcriptional activity [119] by increasing AR degradation in a complex that includes Akt, Mdm2, and the AR [122] | Increased survival of PC when androgen favors PC apoptosis | |
AR and Signal Transduction Pathways
Steroid receptors are phosphoproteins. Phosphorylation of human steroid hormone receptors is generally believed to positively or negatively modify their transcriptional activity rather than act as an on-off switch. Phosphorylation-dephosphorylation events are influenced by ligand binding, which may affect both ligand-dependent and ligand-independent receptor functions [74]. Such events may predominantly serve to fine-tune aspects of receptor regulation, perhaps by influencing the integration of signals from other pathways or by modulating the subcellular localization, trafficking, and degradation of receptor proteins [74–76]. Analogous to other members of the steroid receptor superfamily, AR is highly phosphorylated [77] and therefore sensitive to growth factor-initiated signalling pathways. Site-directed mutagenesis has confirmed three phosphorylation sites on the AR: two in the N-terminal domain (Ser81, Ser94) and one in the hinge region (Ser650) [78]. Identification of these AR phosphorylation sites allows the study of protein kinase(s), which may be involved in the phosphorylation of AR under conditions of androgen ablation.
MAPKs
MAPKs are mediators of cellular responses to many extracellular stimuli [79]. At least three subgroups have been identified: extracellular signal-regulated kinase (ERK), c-Jun N-terminal protein kinase (JNK), also known as stress-activated protein kinase (SAPK), and p38/HOG (reactivating protein kinase). These three MAPK subfamilies represent similar, yet distinct protein kinase cascades. Each consists of a module of three cytoplasmic kinases: a MAPK kinase kinase (MAPKKK), a MAPK kinase (MAPKK), and the MAPK itself [80–82].
The best understood MAPK signal transduction pathway in mammalian cells is that formed by the Raf/MEK/ERK. Proliferative signals provided by growth factors activate the MAPK pathway through their cognate receptors. Receptor tyrosine kinase activation and phosphorylation recruit adaptor proteins Grb2 and Sos. Sos is a GTP exchange factor, which activates the small G-protein Ras. This in turn activates the MEK kinase (MEKK) Raf, the MAPK/ERK (MEK) kinase, and subsequently MAPK/ERK [79,82]. ERK activity is increased in many human tumors including PC. Both ERK expression and activation are postulated to mediate important roles in the initiation and progression of PC [83,84]. In the AI PC DU145 cell line, the constitutive phosphorylation of ERK2, a hallmark of MAPK activation has been reported and can be blocked by several EGFR inhibitors [85].
The MAPK pathway is also a target of steroid receptors (Figure 1). In addition to being transcription factors, both estrogen receptor (ER) and AR activate MAPK [86–90]. In a complex with Src/Shc, AR activates ERK-1 and ERK-2 in PC cells to stimulate their proliferation [90]. This rapid effect of the AR (within 2 minutes) [89,91] is distinct from its genotropic effects. It may be effected by less fully developed or broader receptor conformations initiated by brief association with the ligand [91]. In nonprostatic cells, the ligand binding domains of ER and AR were sufficient to stimulate an anti-apoptotic response through Src/Shc/ERK, a response which was eliminated by nuclear targeting of the receptor [91]. The activation of ERK by AR is specific for this class of kinases and cannot be inhibited by antiandrogens [89]. These findings imply that the current therapeutically available antiandrogens may not be totally effective in abrogating all androgenic activities in target cells.
Conversely, MAPK pathways were shown to activate the AR and thus mimic androgen signalling in the androgen-depleted environment [92]. MAPK was required for both ligand-dependent and ligand-independent activation of the AR downstream of ErbB2 and IL-6 [65,66,93]. ErbB2 was shown to induce androgen-dependent gene activation in the absence of ligand through the phosphorylation of AR in the N-terminal segment at amino acids 511–515 by MAPK (ERK2) [66]. The phosphorylation of steroid receptor coactivator-1 (SRC-1) by MAPK was also required for optimal ligand-independent activation of the AR by IL-6 [93].
In contrast to the Raf/MEK/ERK pathway, MEKKs comprise a family of related serine-threonine protein kinases that regulate MAPK signalling pathways, leading to c-Jun N terminal kinase and p38. These MAPK pathways are induced by cellular stress, inflammatory cytokines, and G-protein-coupled protein agonists. They have been implicated in apoptosis, oncogenic transformation, and inflammatory responses in various cell types. MEKK1 mediates Ras-dependent activation of JNK cascades by growth factor receptors through an association with Grb2 [82] (Figure 1). The link between MEKK1 and AR signalling is contradictory and puzzling. The MEKK1 pathway may contribute to the progression of PC to AI by modulating the activation of AR and its transcriptional response to ligands [94]. A constitutively active MEKK1 stimulated natural and artificial androgen response promoter templates in an ARdependent manner and induced transcriptional activation of AR-regulated genes in the absence of androgens. Furthermore, a dominant negative mutant of MEKK1 impaired the activation of the AR by androgen [94]. The molecular basis of this crosstalk is unclear. MEKK1 possibly activates signalling cascades (such as c-Jun) that indirectly lead to posttranslational modifications of the AR and affect its function. Homodimerization of the AR and other steroid receptors is required for these transcription factors to bind DNA. This homodimerization is thought to result from an intramolecular or intermolecular interaction between the amino and carboxyl termini of the receptor [95]. The downstream target of MEKK1/JNK, c-Jun, interacts with the DNA binding domain/hinge region of AR to potentiate the functional interaction between N-terminal and C-terminal and enhance AR DNA binding and gene transcription [96,97].
MEKK1 induced apoptosis in diverse non-AR-expressing cells in response to stress or DNA-damaging agents [82]. Interestingly in PC, MEKK1 induces an apoptotic effect only when the AR signalling pathway is intact [94]. The requirement for AR in mediating the apoptotic effect of MEKK1 apparently contrasted with the role of AR signalling as a survival and/or proliferative factor in prostate secretory epithelial cells. However, AR signalling has been detrimental to PC growth and survival in certain settings (e.g., the effects of high concentrations of androgen on the AR-positive LNCaP cells or transfection of AR into AR-negative PC3 and DU145 cells) [98,99].
PI3K
Phosphorylation of phosphatidylinositol (PtdIn) at the D3 position by extracellular stimuli plays a major role in cell survival. Through activation of protein kinases such as the phosphoinositide-dependent protein kinases (PDK1 and PDK2) and Akt/protein kinase B (Akt/PKB), PtdIn 3,4,5-trisphosphate (PIP3) inhibits apoptosis and influences other intracellular metabolic functions. Targets phosphorylated by Akt include the proapoptotic proteins (e.g., BAD), caspase 9, and the forkhead transcription factors as well as enzymes involved in intracellular metabolism such as glycogen synthase kinase-3 (GSK-3β) [100–102] (Figure 1B). PTEN (phosphatase and tensin homologue deleted on chromosome 10) is the negative downregulator of the PI3K pathway acting as a PtdIn phosphatase. Loss of PTEN function results in the constitutive activation of Akt. Tumor cells affected in this manner may escape dependence on extracellular survival factors and may become more resistant to agents that induce apoptosis [103]. The increased frequency of PTEN dysfunction and/or mutations in prostate and other cancers underscores the potential importance of signals provided by the PI3K/Akt pathway in the development and proliferation of cancer cells [104–106].
The PI3K/Akt signalling axis has been described recently as a dominant growth factor survival pathway in PC, which at best can only be partially compensated by a MAPK-sensitive step [107,108]. In the LNCaP PC model of AI, PI3K signalling was activated at the onset of androgen deprivation and progressively increased thereafter [107,109]. Androgen ablation increased PI3K/Akt activation, possibly by promoting the abnormal establishment of autocrine growth factor loops and/or enhancing the engagement of already-present growth factor signalling pathways [6,110] (Figure 2). PI3K promoted survival of the acute effects of androgen deprivation and promoted proliferation of AI PC cells by diminishing the expression of the cyclin-dependent inhibitor p27kip1 [111] and/or increasing its degradation [107]. ErbB2-induced AR transactivation is mediated only partially through the MAPK pathway and requires a functional PI3K/Akt pathway [109]. Inhibition of PI3K signalling by dominant negative Akt or the PI3K inhibitor LY294002 completely abolished AR activation by ErbB2, whereas PD98059, an MAPK inhibitor, inhibited it only partially [66,109]. Attenuation of the PI3K/Akt pathway either by pharmacological inhibitors or PTEN triggered a rapid and extensive apoptosis in LNCaP cells [107,108,112,113]. This response could not achieved by MAPK inhibition and was prevented by pretreatment with androgens or growth factors [107,108,112,114]. The mechanism by which androgens can overcome the apoptotic response to PI3K inhibition is yet unknown. It may result from PI3K activation and/or PTEN inhibition and it clearly involves the AR [108,114]. Some researchers demonstrated that in AR-positive cells, DHT rapidly activated PI3K and increased intracellular PIP3 in an in vitro kinase assay (Figure 1B) [89,115], whereas others failed to observe any effect of DHT on PI3K or PTEN [114,116]. Alternatively, androgens may protect from PTEN-induced apoptosis either by activating a survival pathway that is independent of Akt, or by activating the same survival pathway at steps downstream of Akt [112,116]. PI3K/Akt has been shown to be a key regulator of AR expression in mouse vas deferens epithelial cells and in LNCaP cells. Inhibition of the PI3K pathway strongly decreased both basal and DHT-induced levels of AR [117]. Exposure of LNCaP to LY294002 pretreatment inhibited the increased expression of PSA mRNA normally induced by DHT [117,118].
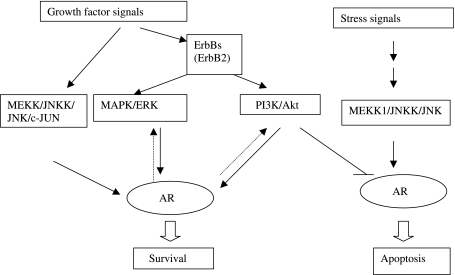
Figure 2.
A synopsis of the crosstalk between AR and the ErbB cascade. This figure provides a brief outline of the interplay of AR and ErbB cascade in promoting PC survival. Growth factor signals augment AR survival signals and enhance AR transactivation through the MAPK/ERK, MEKK/JNKK/c-Jun, and the PI3K/Akt pathways. This enhancement is shown in vitro to occur both in the presence and absence of ligand. Apoptotic responses initiated by stress signals acting through MEKK/JNKK/JNK and the AR can be subverted by activation of the PI3K/Akt pathway downstream of growth factors, thereby allowing PC cell survival.
Protein Kinase B/Akt
Transfection of a constitutively active Akt maintains PC survival in an androgen-poor or growth factor-poor environment [107,111]. In addition to its negative effects on the proapoptotic machinery, some studies have demonstrated that activated Akt specifically interacted with and phosphorylated both ERα (at Ser167) and AR (two sites): N-terminal Ser210 and C-terminal Ser790 [109–121]. AR phosphorylation by Akt was shown to promote PC survival, but whether it activated or inhibited AR gene transcription was unclear. Several groups have reported the activation of the AR transcription by Akt in the absence of androgen [109,114]. In support of the above findings, PTEN, the negative regulator of the PI3K pathway, antagonized AR signalling and androgen-induced cell proliferation and repressed the transcriptional activity of the AR as well as PSA production [114]. In contrast, Lin et al. [119] reported that Akt inhibited androgen/AR-induced apoptosis and blocked AR target genes such as p21, thereby promoting PC survival. The constitutively active forms of PI3K or Akt repressed the transactivation of AR and the interaction between AR and its coactivators. Additionally, the phosphorylation of AR by Akt appears to be critical for AR ubiquitylation and subsequent degradation by the proteasome [122]. This effect of Akt on AR was markedly reduced if two serine residues (Ser210 and Ser790) were replaced with alanine, preventing Akt induced AR phosphorylation, or in a Mdm2-null cell line compared with the wild-type cell line [122]. Other researchers have been unable to demonstrate a physical protein-protein interaction between Akt and AR or phosphorylation of AR by Akt in vitro [114,118]. They proposed that β-catenin acted as a mediator in the crosstalk between PI3K and androgen signalling [118]. PI3K/Akt induced the phosphorylation and inactivation of GSK-3β, which in turn increased nuclear levels of β-catenin. Increased β-catenin elevates AR activity, stimulating PC growth and survival [118].
The interaction between the AR and the PI3K/Akt pathway is summarized in Figure 1B and in Table 1.
Conclusion
The development of AI PC is one of the major problems in its treatment. A shift in growth support from androgens to growth factors may enhance the ability of PC cells to survive the acute effects of androgen withdrawal and to proliferate in androgen-depleted conditions. The continued expression of AR and AR-regulated genes in AI PC suggests that alternative signalling pathways are utilized to activate the AR. Elements of the AR and ErbB pathways interact, cross over, and converge on targets downstream of each other's signalling cascades to promote AI PC cell survival (summarized in Table 1 and Figure 2). It is noteworthy to mention that studies revealing AI mechanisms of AR activation have been performed in vitro. Additionally and more importantly, the ligand-free environment that is achievable in the laboratory may not be reflected in the clinical situation because very low levels of androgen remain in patients' serum (castrate testosterone is defined as less than 50 ng/dl) despite surgical or pharmacologic castration.
Although manipulative in vitro data or descriptive in vivo data have not yet resolved the mechanism of AI growth, identification of these interactions and their physiological relevance enhances understanding of the possible means by which PC cells escape their requirement for androgen. This understanding suggests that inhibition of these signalling interactions could convert AI PC back to a hormonesensitive state and offers possible additional strategies in the management of PC.
Abbreviations
- AI
androgen independence
- AR
androgen receptor
- MAB
maximal androgen blockade
- PIP3
3′-phosphoinositides
- PC
prostate cancer
References
- 1.Majeed A, Babb P, Jones J, Quinn M. Trends in prostate cancer incidence, mortality and survival in England and Wales 1971–1998. BJU Int. 2000;85:1058–1062. doi: 10.1046/j.1464-410x.2000.00661.x. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Lalani E-N, Laniado ME, Abel PD. Molecular and cellular biology of prostate cancer. Cancer Metastasis Rev. 1997;16:29–66. doi: 10.1023/a:1005792206377. [DOI] [PubMed] [Google Scholar]
- 4.Westin P, Bergh A. Apoptosis and other mechanisms in androgen ablation treatment and androgen-independent progression of prostate cancer: a review. Cancer Detect Prev. 1998;22:476–484. [PubMed] [Google Scholar]
- 5.Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON, Said J, Reiter RE, Sawyers CL. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 1999;59:5030–5036. [PubMed] [Google Scholar]
- 6.Russell PJ, Bennett S, Stricker P. Growth factor involvement in progression of prostate cancer. Clin Chem. 1998;44:705–723. [PubMed] [Google Scholar]
- 7.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 8.Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 9.Fang Y, Fliss AE, Robins DM, Caplan AJ. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem. 1996;271:28697–28702. doi: 10.1074/jbc.271.45.28697. [DOI] [PubMed] [Google Scholar]
- 10.Haendler B, Schuttke I, Schleuning WD. Androgen receptor signalling: comparative analysis of androgen response elements and implication of heat-shock protein 90 and 14-3-3eta. Mol Cell Endocrinol. 2001;173:63–73. doi: 10.1016/s0303-7207(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 11.Wong CI, Zhou ZX, Sar M, Wilson EM. Steroid requirement for androgen receptor dimerization and DNA binding. Modulation by intramolecular interactions between the NH2-terminal and steroid-binding domains. J Biol Chem. 1993;268:19004–19012. [PubMed] [Google Scholar]
- 12.Kemppainen JA, Lane MV, Sar M, Wilson EM. Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. J Biol Chem. 1992;267:968–974. [PubMed] [Google Scholar]
- 13.Kang HY, Yeh S, Fujimoto N, Chang C. Cloning and characterization of human prostate coactivator ARA54, a novel protein that associates with the androgen receptor. J Biol Chem. 1999;274:8570–8576. doi: 10.1074/jbc.274.13.8570. [DOI] [PubMed] [Google Scholar]
- 14.Gnanapragasam VJ, Robson CN, Leung HY, Neal DE. Androgen receptor signalling in the prostate. BJU Int. 2000;86:1001–1013. doi: 10.1046/j.1464-410x.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 15.Heidenberg HB, Sesterhenn IA, Gaddipati JP, Weghorst CM, Buzard GS, Moul JW, Srivastava S. Alteration of the tumor suppressor gene p53 in a high fraction of hormone refractory prostate cancer. J Urol. 1995;154:414–421. doi: 10.1097/00005392-199508000-00024. [DOI] [PubMed] [Google Scholar]
- 16.DiPaola RS, Aisner J. Overcoming bcl-2- and p53-mediated resistance in prostate cancer. Semin Oncol. 1999;26:112–116. [PubMed] [Google Scholar]
- 17.Moul JW. Angiogenesis, p53, bcl-2 and Ki-67 in the progression of prostate cancer after radical prostatectomy. Eur Urol. 1999;35:399–407. doi: 10.1159/000019916. [DOI] [PubMed] [Google Scholar]
- 18.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 19.Koivisto P, Hyytinen E, Palmberg C, Tammela T, Visakorpi T, Isola J, Kallioniemi OP. Analysis of genetic changes underlying local recurrence of prostate carcinoma during androgen deprivation therapy. Am J Pathol. 1995;147:1608–1614. [PMC free article] [PubMed] [Google Scholar]
- 20.El Gedaily A, Bubendorf L, Willi N, Fu W, Richter J, Moch H, Mihatsch MJ, Sauter G, Gasser TC. Discovery of new DNA amplification loci in prostate cancer by comparative genomic hybridization. Prostate. 2001;46:184–190. doi: 10.1002/1097-0045(20010215)46:3<184::aid-pros1022>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 22.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 23.Palmberg C, Koivisto P, Hyytinen E, Isola J, Visakorpi T, Kallioniemi OP, Tammela T. Androgen receptor gene amplification in a recurrent prostate cancer after monotherapy with the nonsteroidal potent antiandrogen Casodex (bicalutamide) with a subsequent favorable response to maximal androgen blockade. Eur Urol. 1997;31:216–219. doi: 10.1159/000474453. [DOI] [PubMed] [Google Scholar]
- 24.Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–2898. [PubMed] [Google Scholar]
- 25.Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, Gumerlock PH, de Vere White RW, Pretlow TG, Harris SE, Wilson EM, Mohler JL, French FS. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11:450–459. doi: 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- 26.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 27.Haapala K, Hyytinen ER, Roiha M, Laurila M, Rantala I, Helin HJ, Koivisto PA. Androgen receptor alterations in prostate cancer relapsed during a combined androgen blockade by orchiectomy and bicalutamide. Lab Invest. 2001;81:1647–1651. doi: 10.1038/labinvest.3780378. [DOI] [PubMed] [Google Scholar]
- 28.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, Balk SP. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–2515. [PubMed] [Google Scholar]
- 29.Gaddipati JP, McLeod DG, Heidenberg HB, Sesterhenn IA, Finger MJ, Moul JW, Srivastava S. Frequent detection of codon 877 mutation in the androgen receptor gene in advanced prostate cancers. Cancer Res. 1994;54:2861–2864. [PubMed] [Google Scholar]
- 30.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 31.Moul JW, Srivastava S, McLeod DG. Molecular implications of the antiandrogen withdrawal syndrome. Semin Urol. 1995;13:157–163. [PubMed] [Google Scholar]
- 32.Miyamoto H, Yeh S, Wilding G, Chang C. Promotion of agonist activity of antiandrogens by the androgen receptor coactivator, ARA70, in human prostate cancer DU145 cells. Proc Natl Acad Sci USA. 1998;95:7379–7384. doi: 10.1073/pnas.95.13.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–4319. [PubMed] [Google Scholar]
- 34.Yeh S, Chang HC, Miyamoto H, Takatera H, Rahman M, Kang HY, Thin TH, Lin HK, Chang C. Differential induction of the androgen receptor transcriptional activity by selective androgen receptor coactivators. Keio J Med. 1999;48:87–92. doi: 10.2302/kjm.48.87. [DOI] [PubMed] [Google Scholar]
- 35.Barton J, Blackledge G, Wakeling A. Growth factors and their receptors: new targets for prostate cancer therapy. Urology. 2001;58:114–122. doi: 10.1016/s0090-4295(01)01253-5. [DOI] [PubMed] [Google Scholar]
- 36.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 37.Culig Z, Hobisch A, Cronauer MV, Hittmair A, Radmayr C, Bartsch G, Klocker H. Activation of the androgen receptor by polypeptide growth factors and cellular regulators. World J Urol. 1995;13:285–289. doi: 10.1007/BF00185971. [DOI] [PubMed] [Google Scholar]
- 38.Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- 39.Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr-Relat Cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- 40.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 41.Kim HG, Kassis J, Souto JC, Turner T, Wells A. EGF receptor signaling in prostate morphogenesis and tumorigenesis. Histol Histopathol. 1999;14:1175–1182. doi: 10.14670/HH-14.1175. [DOI] [PubMed] [Google Scholar]
- 42.Gregory H, Willshire IR, Kavanagh JP, Blacklock NJ, Chowdury S, Richards RC. Urogastrone-epidermal growth factor concentrations in prostatic fluid of normal individuals and patients with benign prostatic hypertrophy. Clin Sci (London) 1986;70:359–363. doi: 10.1042/cs0700359. [DOI] [PubMed] [Google Scholar]
- 43.De Miguel P, Royuela M, Bethencourt R, Ruiz A, Fraile B, Paniagua R. Immunohistochemical comparative analysis of transforming growth factor alpha, epidermal growth factor, and epidermal growth factor receptor in normal, hyperplastic and neoplastic human prostates. Cytokine. 1999;11:722–727. doi: 10.1006/cyto.1998.0443. [DOI] [PubMed] [Google Scholar]
- 44.Kumar VL, Majumder PK, Gujral S, Kumar V. Comparative analysis of epidermal growth factor receptor mRNA levels in normal, benign hyperplastic and carcinomatous prostate. Cancer Lett. 1998;134:177–180. doi: 10.1016/s0304-3835(98)00256-0. [DOI] [PubMed] [Google Scholar]
- 45.Harper ME, Glynne-Jones E, Goddard L, Mathews P, Nicholson RI. Expression of androgen receptor and growth factors in premalignant lesions of the prostate. J Pathol. 1998;186:169–177. doi: 10.1002/(SICI)1096-9896(1998100)186:2<169::AID-PATH164>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 46.Connolly JM, Rose DP. Autocrine regulation of DU145 human prostate cancer cell growth by epidermal growth factor-related polypeptides. Prostate. 1991;19:173–180. doi: 10.1002/pros.2990190210. [DOI] [PubMed] [Google Scholar]
- 47.Myers RB, Oelschlager D, Manne U, Coan PN, Weiss H, Grizzle WE. Androgenic regulation of growth factor and growth factor receptor expression in the CWR22 model of prostatic adenocarcinoma. Int J Cancer. 1999;82:424–429. doi: 10.1002/(sici)1097-0215(19990730)82:3<424::aid-ijc16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 48.Ye D, Mendelsohn J, Fan Z. Androgen and epidermal growth factor down-regulate cyclin-dependent kinase inhibitor p27Kip1 and costimulate proliferation of MDA PCa 2a and MDA PCa 2b prostate cancer cells. Clin Cancer Res. 1999;5:2171–2177. [PubMed] [Google Scholar]
- 49.Liu XH, Wiley HS, Meikle AW. Androgens regulate proliferation of human prostate cancer cells in culture by increasing transforming growth factor-alpha, (TGF-alpha) and epidermal growth factor, (EGF)/TGF-alpha receptor. J Clin Endocrinol Metab. 1993;77:1472–1478. doi: 10.1210/jcem.77.6.8263129. [DOI] [PubMed] [Google Scholar]
- 50.Brass AL, Barnard J, Patai BL, Salvi D, Rukstalis DB. Androgen up-regulates epidermal growth factor receptor expression and binding affinity in PC3 cell lines expressing the human androgen receptor. Cancer Res. 1995;55:3197–3203. [PubMed] [Google Scholar]
- 51.Ravenna L, Lubrano C, Di Silverio F, Vacca A, Felli MP, Maroder M, D'Eramo G, Sciarra F, Frati L, Gulino A, et al. Androgenic and antiandrogenic control on epidermal growth factor, epidermal growth factor receptor, and androgen receptor expression in human prostate cancer cell line LNCaP. Prostate. 1995;26:290–298. doi: 10.1002/pros.2990260604. [DOI] [PubMed] [Google Scholar]
- 52.Bostwick DG. Target populations and strategies for chemoprevention trials of prostate cancer. J Cell Biochem Suppl. 1994;19:191–196. [PubMed] [Google Scholar]
- 53.Bostwick DG, Aquilina JW. Prostatic intraepithelial neoplasia (PIN) and other prostatic lesions as risk factors and surrogate endpoints for cancer chemoprevention trials. J Cell Biochem Suppl. 1996;25:156–164. [PubMed] [Google Scholar]
- 54.Ross JS, Sheehan CE, Hayner-Buchan AM, Ambros RA, Kallakury BV, Kaufman RP, Jr, Fisher HA, Rifkin MD, Muraca PJ. Prognostic significance of HER-2/neu gene amplification status by fluorescence in situ hybridization of prostate carcinoma. Cancer. 1997;79:2162–2170. doi: 10.1002/(sici)1097-0142(19970601)79:11<2162::aid-cncr14>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 55.Reese DM, Small EJ, Magrane G, Waldman FM, Chew K, Sudilovsky D. HER2 protein expression and gene amplification in androgen-independent prostate cancer. Am J Clin Pathol. 2001;116:234–239. doi: 10.1309/VXKK-YVRH-9B11-YDPT. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe M, Nakada T, Yuta H. Analysis of protooncogene c-erbB-2 in benign and malignant human prostate. Int Urol Nephrol. 1999;31:61–73. doi: 10.1023/a:1007123807244. [DOI] [PubMed] [Google Scholar]
- 57.Osman I, Scher HI, Drobnjak M, Verbel D, Morris M, Agus D, Ross JS, Cordon-Cardo C. HER-2/neu (p185neu) protein expression in the natural or treated history of prostate cancer. Clin Cancer Res. 2001;7:2643–2647. [PubMed] [Google Scholar]
- 58.Morote J, de Torres I, Caceres C, Vallejo C, Schwartz S, Jr, Reventos J. Prognostic value of immunohistochemical expression of the c-erbB-2 oncoprotein in metastasic prostate cancer. Int J Cancer. 1999;84:421–425. doi: 10.1002/(sici)1097-0215(19990820)84:4<421::aid-ijc16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 59.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, Hahnfeldt P, Kantoff P, Loda M. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 60.Visakorpi T, Kallioniemi OP, Koivula T, Harvey J, Isola J. Expression of epidermal growth factor receptor and ERBB2 (HER-2/Neu) oncoprotein in prostatic carcinomas. Mod Pathol. 1992;5:643–648. [PubMed] [Google Scholar]
- 61.Savinainen KJ, Saramaki OR, Linja MJ, Bratt O, Tammela TL, Isola JJ, Visakorpi T. Expression and gene copy number analysis of ERBB2 oncogene in prostate cancer. Am J Pathol. 2002;160:339–345. doi: 10.1016/S0002-9440(10)64377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, Willi N, Mihatsch MJ, Sauter G, Kallioniemi OP. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59:803–806. [PubMed] [Google Scholar]
- 63.Arai Y, Yoshiki T, Yoshida O. c-erbB-2 oncoprotein: a potential biomarker of advanced prostate cancer. Prostate. 1997;30:195–201. doi: 10.1002/(sici)1097-0045(19970215)30:3<195::aid-pros8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 64.Myers RB, Brown D, Oelschlager DK, Waterbor JW, Marshall ME, Srivastava S, Stockard CR, Urban DA, Grizzle WE. Elevated serum levels of p105(erbB-2) in patients with advanced-stage prostatic adenocarcinoma. Int J Cancer. 1996;69:398–402. doi: 10.1002/(SICI)1097-0215(19961021)69:5<398::AID-IJC8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 65.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 66.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 68.Qiu Y, Ravi L, Kung HJ. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature. 1998;393:83–85. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- 69.Gullick WJ. The c-erbB3/HER3 receptor in human cancer. Cancer Surv. 1996;27:339–349. [PubMed] [Google Scholar]
- 70.Lyne JC, Melhem MF, Finley GG, Wen D, Liu N, Deng DH, Salup R. Tissue expression of neu differentiation factor/heregulin and its receptor complex in prostate cancer and its biologic effects on prostate cancer cells in vitro. Cancer J Sci Am. 1997;3:21–30. [PubMed] [Google Scholar]
- 71.Grasso AW, Wen D, Miller CM, Rhim JS, Pretlow TG, Kung HJ. ErbB kinases and NDF signaling in human prostate cancer cells. Oncogene. 1997;15:2705–2716. doi: 10.1038/sj.onc.1201447. [DOI] [PubMed] [Google Scholar]
- 72.Leung HY, Weston J, Gullick WJ, Williams G. A potential autocrine loop between heregulin-alpha and erbB-3 receptor in human prostatic adenocarcinoma. Br J Urol. 1997;79:212–216. doi: 10.1046/j.1464-410x.1997.30412.x. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Fondell JD, Wang Q, Xia X, Cheng A, Lu ML, Hamburger AW. Repression of androgen receptor mediated transcription by the ErbB-3 binding protein, Ebp1. Oncogene. 2002;21:5609–5618. doi: 10.1038/sj.onc.1205638. [DOI] [PubMed] [Google Scholar]
- 74.Blok LJ, de Ruiter PE, Brinkmann AO. Androgen receptor phosphorylation. Endocr Res. 1996;22:197–219. doi: 10.3109/07435809609030508. [DOI] [PubMed] [Google Scholar]
- 75.Weigel NL. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319(Part 3):657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuiper GG, Brinkmann AO. Steroid hormone receptor phosphorylation: is there a physiological role? Mol Cell Endocrinol. 1994;100:103–107. doi: 10.1016/0303-7207(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 77.van Laar JH, Bolt-de Vries J, Zegers ND, Trapman J, Brinkmann AO. Androgen receptor heterogeneity and phosphorylation in human LNCaP cells. Biochem Biophys Res Commun. 1990;166:193–200. doi: 10.1016/0006-291x(90)91930-q. [DOI] [PubMed] [Google Scholar]
- 78.Zhou ZX, Kemppainen JA, Wilson EM. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol Endocrinol. 1995;9:605–615. doi: 10.1210/mend.9.5.7565807. [DOI] [PubMed] [Google Scholar]
- 79.Brunet A, Brondello JM, L'Allemain G, Lenormand P, McKenzie F, Pages G, Pouyssegur J. MAP kinase module: role in the control of cell proliferation. C R Seances Soc Biol Fil. 1995;189:43–57. [PubMed] [Google Scholar]
- 80.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 81.Kyriakis JM. Making the connection: coupling of stress-activated ERK/MAPK (extracellular-signal-regulated kinase/mitogen-activated protein kinase) core signalling modules to extracellular stimuli and biological responses. Biochem Soc Symp. 1999;64:29–48. [PubMed] [Google Scholar]
- 82.Hagemann C, Blank JL. The ups and downs of MEK kinase interactions. Cell Signal. 2001;13:863–875. doi: 10.1016/s0898-6568(01)00220-0. [DOI] [PubMed] [Google Scholar]
- 83.Cobb MH, Hepler JE, Cheng M, Robbins D. The mitogen-activated protein kinases, ERK1 and ERK2. Semin Cancer Biol. 1994;5:261–268. [PubMed] [Google Scholar]
- 84.Price DT, Rocca GD, Guo C, Ballo MS, Schwinn DA, Luttrell LM. Activation of extracellular signal-regulated kinase in human prostate cancer. J Urol. 1999;162:1537–1542. [PubMed] [Google Scholar]
- 85.Putz T, Culig Z, Eder IE, Nessler-Menardi C, Bartsch G, Grunicke H, Uberall F, Klocker H. Epidermal growth factor (EGF) receptor blockade inhibits the action of EGF, insulin-like growth factor I, and a protein kinase A activator on the mitogen-activated protein kinase pathway in prostate cancer cell lines. Cancer Res. 1999;59:227–233. [PubMed] [Google Scholar]
- 86.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 87.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu X, Li H, Liu JP, Funder JW. Androgen stimulates mitogen-activated protein kinase in human breast cancer cells. Mol Cell Endocrinol. 1999;152:199–206. doi: 10.1016/s0303-7207(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 89.Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–6329. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- 90.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 92.Zhu X, Liu JP. Steroid-independent activation of androgen receptor in androgen-independent prostate cancer: a possible role for the MAP kinase signal transduction pathway? Mol Cell Endocrinol. 1997;134:9–14. doi: 10.1016/s0303-7207(97)00168-8. [DOI] [PubMed] [Google Scholar]
- 93.Ueda T, Mawji NR, Bruchovsky N, Sadar MD. Ligand-independent activation of the androgen receptor by IL-6 and the role of the coactivator SRC-1 in prostate cancer cells. J Biol Chem. 2002;277(41):38087–38094. doi: 10.1074/jbc.M203313200. [DOI] [PubMed] [Google Scholar]
- 94.Abreu-Martin MT, Chari A, Palladino AA, Craft NA, Sawyers CL. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol. 1999;19:5143–5154. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doesburg P, Kuil CW, Berrevoets CA, Steketee K, Faber PW, Mulder E, Brinkmann AO, Trapman J. Functional in vivo interaction between the amino-terminal, transactivation domain and the ligand binding domain of the androgen receptor. Biochemistry. 1997;36:1052–1064. doi: 10.1021/bi961775g. [DOI] [PubMed] [Google Scholar]
- 96.Bubulya A, Wise SC, Shen XQ, Burmeister LA, Shemshedini L. c-Jun can mediate androgen receptor-induced transactivation. J Biol Chem. 1996;271:24583–24589. doi: 10.1074/jbc.271.40.24583. [DOI] [PubMed] [Google Scholar]
- 97.Bubulya A, Zhou XF, Shen XQ, Fisher CJ, Shemshedini L. c-Jun targets amino terminus of androgen receptor in regulating androgen-responsive transcription. Endocrine. 2000;13:55–62. doi: 10.1385/ENDO:13:1:55. [DOI] [PubMed] [Google Scholar]
- 98.Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 99.Mitchell S, Abel P, Madaan S, Jeffs J, Chaudhary K, Stamp GW, Lalani EN. Androgen dependent regulation of human MUC1 mucin expression. Neoplasia. 2002;4:9–18. doi: 10.1038/sj.neo.7900194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 101.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 102.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 103.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 105.Facher EA, Law JC. PTEN and prostate cancer. J Med Genet. 1998;35:790. doi: 10.1136/jmg.35.9.790-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Whang YE, Wu X, Suzuki H, Reiter RE, Tran C, Vessella RL, Said JW, Isaacs WB, Sawyers CL. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA. 1998;95:5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–4805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 108.Lin J, Adam RM, Santiestevan E, Freeman MR. The phosphatidylinositol 3′-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res. 1999;59:2891–2897. [PubMed] [Google Scholar]
- 109.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 110.Nelson JB, Carducci MA. Small bioactive peptides and cell surface peptidases in androgen-independent prostate cancer. Cancer Invest. 2000;18:87–96. doi: 10.3109/07357900009023066. [DOI] [PubMed] [Google Scholar]
- 111.Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE, Vessella RL, Neubauer BL. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275:24500–24505. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 112.Carson JP, Kulik G, Weber MJ. Antiapoptotic signaling in LNCaP prostate cancer cells: a survival signaling pathway independent of phosphatidylinositol 3′-kinase and Akt/protein kinase B. Cancer Res. 1999;59:1449–1453. [PubMed] [Google Scholar]
- 113.Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, Yung WK, Steck PA. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551–2556. [PubMed] [Google Scholar]
- 114.Li P, Nicosia SV, Bai W. Antagonism between PTEN/MMAC1/TEP-1 and androgen receptor in growth and apoptosis of prostatic cancer cells. J Biol Chem. 2001;276:20444–20450. doi: 10.1074/jbc.M010226200. [DOI] [PubMed] [Google Scholar]
- 115.Wilding G, Gelmann EP, Freter CE. Phosphoinositide metabolism in human prostate cancer cells in vitro. Prostate. 1990;16:15–27. doi: 10.1002/pros.2990160103. [DOI] [PubMed] [Google Scholar]
- 116.Kimura K, Markowski M, Bowen C, Gelmann EP. Androgen blocks apoptosis of hormone-dependent prostate cancer cells. Cancer Res. 2001;61:5611–5618. [PubMed] [Google Scholar]
- 117.Manin M, Baron S, Goossens K, Beaudoin C, Jean C, Veyssiere G, Verhoeven G, Morel L. Androgen receptor expression is regulated by the PI3-kinase/Akt pathway in normal and tumoral epithelial cells. Biochem J. 2002;366(Pt 3):729–736. doi: 10.1042/BJ20020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sharma M, Chuang WW, Sun Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J Biol Chem. 2002;277(34):30935–30941. doi: 10.1074/jbc.M201919200. [DOI] [PubMed] [Google Scholar]
- 119.Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci USA. 2001;98:7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 121.Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV, Cheng JQ. Phosphatidylinositol-3-OH kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61:5985–5991. [PubMed] [Google Scholar]
- 122.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]