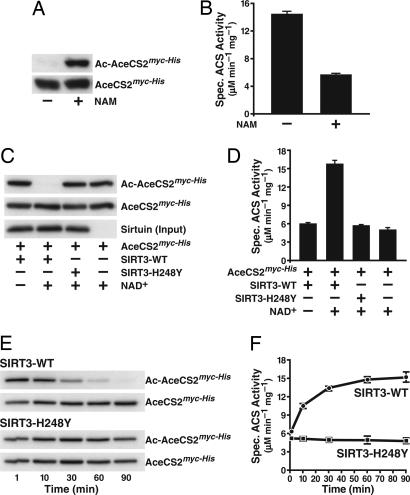
Fig. 4.
Acetylation of Lys-642 controls the acetyl-CoA synthetase activity of human AceCS2. (A) Recombinant human AceCS2 was expressed in bacteria in the presence or absence of NAM. Acetylation levels and total amounts of AceCS2 were analyzed by immunoblotting with acetyl-lysine-specific antibodies or α-myc antibodies. (B) Specific activity of purified AceCS2 expressed in the presence or absence of NAM. The data are means + SD from three independent experiments. (C) NAD+-dependent deacetylation of AceCS2 by SIRT3 but not by SIRT3-H248Y. The bottom blot indicates that equal amounts of SIRT3 or SIRT3-H248Y were added to the deacetylation reactions. (D) Deacetylation of AceCS2 by SIRT3 activates its acetyl-CoA synthetase activity. Equal amounts of AceCS2 were incubated in the absence or presence of SIRT3 or a catalytically inactive SIRT3 mutant (SIRT3-H248Y). Where indicated, NAD+ was added during the incubation. The deacetylation reaction was stopped by the addition of NAM, and the specific activity of AceCS2 was determined. Data are means + SD from three independent activity determinations. (E) Time-dependent activation of AceCS2 by SIRT3. Recombinant human AceCS2 purified from NAM-treated bacteria was incubated with recombinant SIRT3 or SIRT3-H248Y. Samples were brought to 32°C, and NAD+ was added. At the indicated time points, aliquots of the reaction were removed, mixed with NAM, and incubated on ice. The immunoblots show the progressive deacetylation of AceCS2 by SIRT3 but not by SIRT3-H248Y. (F) The specific activity of AceCS2 after incubation with recombinant SIRT3 or SIRT3-H248Y for the indicated time was determined. Means ± SD from three independent activity assays are shown.