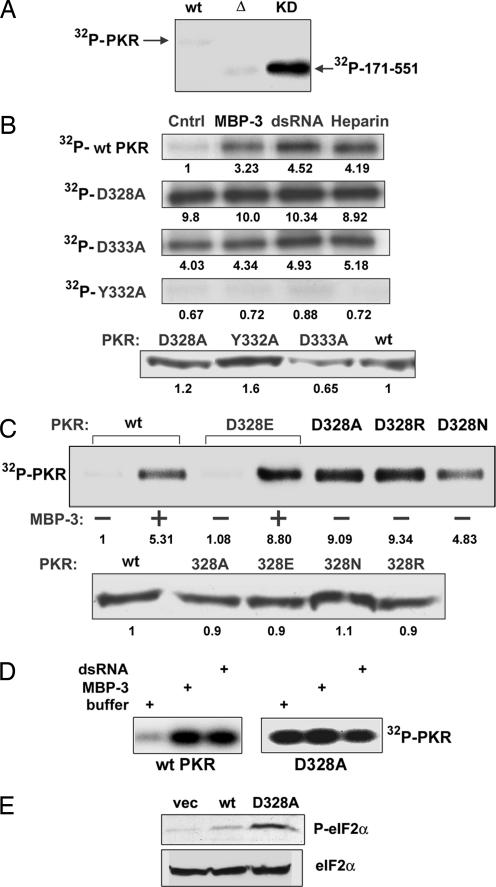
Fig. 3.
Enzymatic activities of PKR mutants as measured by 32P labeling of autophosphorylated proteins. (A) Constitutive activity of the KD. Activities of the isolated KD (residues 171–551) and its mutant were measured in vitro. WT, WT PKR; Δ, the KD missing residues 328–335; KD, the WT KD. (B) Constitutive activation of PKR by mutations of D328 and D333. (Upper) In vitro kinase assays of WT PKR, D328A PKR, D333A PKR, and Y332A PKR were performed in the presence or the absence of MBP-3 (100 nM), dsRNA (200 ng/ml), or heparin (10 units/ml). PKR autophosphorylation was detected by autoradiography. Cntrl, activity buffer. The number at the bottom of each band indicates its relative intensity as measured by PhosphorImager analysis; the intensity of the WT PKR Cntrl was set at 1.0. (Lower) Western blot for PKR and its mutants. The numbers at the bottom are densitometric quantifications of the intensities of the bands. (C) Essential role of the negative charge of D328 in PKR activation. D328 was mutated to alanine, glutamic acid, arginine, or asparagine, and the resultant PKR mutants were examined as in B for their kinase activities (Upper) and protein contents (Lower). (D) Activity of PKR expressed in bacteria. Kinase activities of purified PKR expressed in E. coli were measured in the presence or the absence of the activators. (Left) WT PKR (100 nM). (Right) D328A mutant (100 nM). Where indicated, 100 nM MBP-3 or 200 ng/ml dsRNA was used. (E) Constitutive activity of a PKR mutant in human cells. In vivo action of a constitutively active PKR mutant was tested by expressing it in human cells and measuring the eIF-2α phosphorylation status. (Upper) Western blot with a phospho-eIF-2α-specific antibody. (Lower) Total eIF-2α levels.