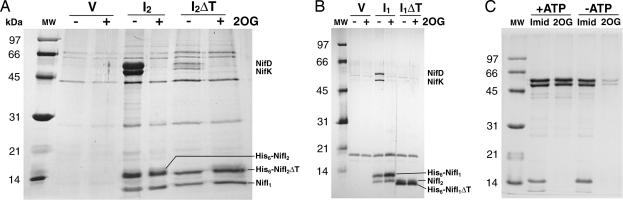
Fig. 1.
Copurification of proteins with His-tagged NifI1 and NifI2 and interaction of NifI1,2 with dinitrogenase. Extracts were bound to Ni-NTA agarose, and elution fractions were analyzed by gel electrophoresis. (A) NifI2 interactions: V, strain Mm1012 [ΔnifI2 (null) background containing pLW40neo (vector control)]; I2, strain Mm711 (ΔnifI2 background containing pLW40neo nifI2 expressing His-tagged NifI2); I2ΔT, strain Mm1017 (ΔnifI2 background containing pLW40neo nifI2ΔT expressing His-tagged NifI2 with a deletion in the T-loop). Extracts were bound to Ni-NTA agarose in the absence (−) or presence (+) of 10 mM 2OG and eluted with 100 mM imidazole. Proteins corresponding to the indicated bands were identified by 2D gel electrophoresis and MALDI-TOF MS. Masses of the molecular weight markers (MW) are shown on the left. (B) NifI1 interactions: V, strain Mm1050 [ΔnifI1 (null) background containing pLCW40neo (vector control)]; I1, strain Mm1051 (ΔnifI1 background containing pLCW40 nifI1 expressing his-tagged NifI1); I1ΔT, strain Mm1067 (ΔnifI1 background containing pLCW40neo nifI1ΔT expressing His-tagged NifI1 with a deletion in the T-loop). Binding and elution are as in A. The band corresponding to NifI2 is partially obscured by His-tagged NifI1ΔT but was separated and identified on 2D gels. (C) Effect of ATP on NifI2 interactions using extract of strain Mm711. Binding, washing, and elution were done in the presence (+) or absence (−) of 5 mM ATP. Elution was done with either 100 mM imidazole (Imid) or 10 mM 2OG. NifI1 is present at the dye front of the gel.