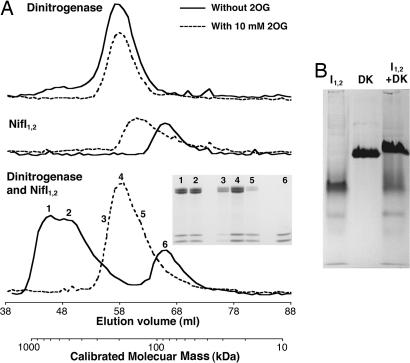
Fig. 2.
Interaction of purified NifI1,2 and dinitrogenase. (A) Gel filtration of dinitrogenase alone, NifI1,2 alone, or a mix of the two was performed anaerobically with ATP and MgCl2, with or without 10 mM 2OG. Protein concentration in fractions, measured as A600 nm, is shown versus the elution volume. The secondary x axis shows the molecular mass corresponding to the fraction volume calibrated by known protein standards. Protein in the fractions indicated by numbers 1–6 were concentrated and run on SDS/PAGE, and the resulting Coomassie-stained gel is shown (Inset). Amounts of protein loaded on the column were dinitrogenase, 1 mg and 2 mg with and without 2OG, respectively; NifI1,2, 0.75 mg and 0.5 mg with and without 2OG, respectively; and dinitrogenase:NifI1,2 mix, 2:1 mg and 3:1.5 mg with and without 2OG, respectively. (B) Purified NifI1,2 (I1,2), dinitrogenase (DK), or a 1:2 mix (NifI1,2/NifDK, by mass) run on an 8% native PAGE gel and stained with Coomassie.