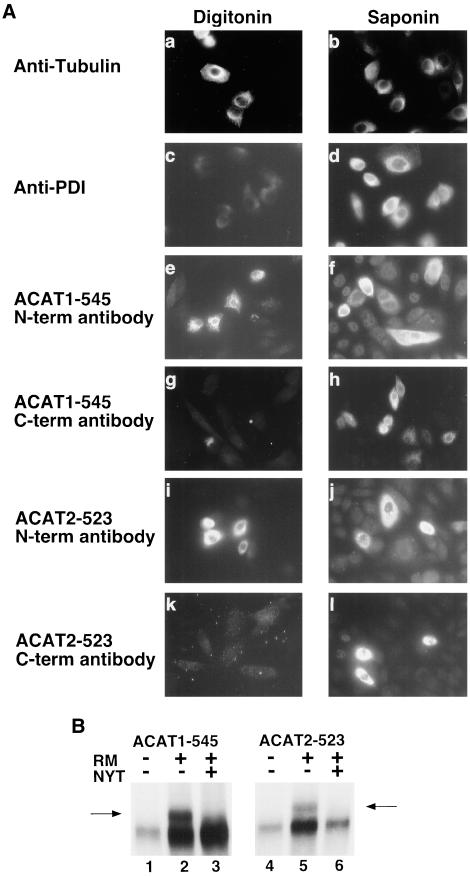
Figure 2.
Orientation of the amino and carboxy termini of ACAT1 and ACAT2 across the ER membrane. (A) Wild-type ACAT-deficient AC29 CHO cells and AC29 cells transiently transfected with either ACAT1-545 or ACAT2-523 (nearly full-length) fusion proteins were treated with digitonin (a, c, e, g, i, and k) to selectively permeabilize the plasma membrane (leaving the ER membrane intact), or with saponin (b, d, f, h, j, and l), to permeabilize all cell membranes. Untransfected cells were treated with digitonin (a and c) or saponin (b and d) and then immunostained with either tubulin (a and b) or PDI (c and d) antibodies. Rhodamine-labeled goat anti-mouse secondary antibody was applied to AC29 cells immunostained with the mouse anti-tubulin antibody and fluorescein-labeled goat anti-rat secondary antibody was applied to AC29 cells immunostained with the rat anti-PDI antibody. Transfected cells were stained with antibodies specific to amino terminal sequences of either ACAT1 (e and f) or ACAT2 (i and j) (N-term antibody) or with the C-terminal-specific (C-term antibody) anti-FLAG M2 antibody (g, h, k, and l). Rhodamine-labeled goat anti-rabbit secondary antibody was applied to cells stained with the ACAT1 or ACAT2 N-term antibodies and rhodamine-labeled goat anti-mouse secondary antibody was applied to cells stained with the anti-FLAG M2 C-term antibody. Cells were viewed microscopically with rhodamine or fluorescein channel fluorescence. Positive signal, where obtained, is indicated by light areas within cells. (B) ACAT1-545 and ACAT2-523 mRNA were translated in rabbit reticulocyte lysate in the presence (+) or absence (−) of canine pancreas rough microsomes (RMs). In lanes 3 and 6, tripeptide glycosylation inhibitor (NYT) was included during the translations. The proteins resulting from each translation were immunoprecipitated by using anti-FLAG M2 antibody and fractionated by 8–20% SDS-polyacrylamide gradient gel electrophoresis. Glycosylated products in lanes 2 and 4 were detected based on their decreased electrophoretic gel mobility (arrows).