Figure 5.
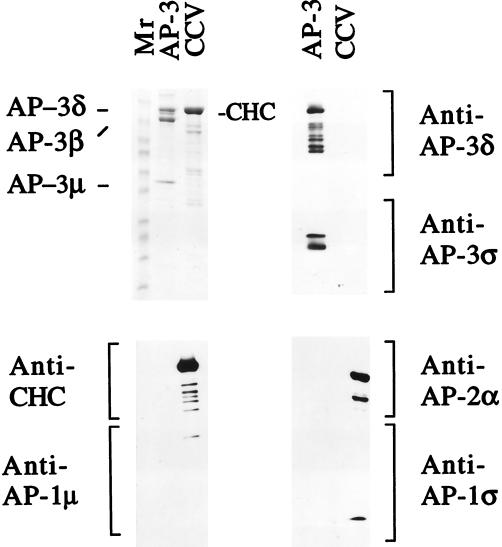
Characterization of the purified AP-3. Ten micrograms of the purified AP-3 material and 35 μg of purified rat brain CCVs were boiled in 50 μl of 1× SDS sample buffer. Thirty-five microliters of each sample was loaded onto a 6–15% gradient gel for Coomassie blue staining, and 5 μl of each sample was loaded in triplicate on the same gel for subsequent immunoblotting with different antibodies. The Coomassie blue staining indicates that the purified AP-3 fraction has only three major bands, labeled AP-3δ, AP-3β, and AP-3μ (upper left panel). Because AP-3δ and the clathrin heavy chain (CHC) comigrate in this gel system, they were identified by immunoblotting. The anti-AP-3δ antibody detected only AP-3δ in the AP-3 fraction (upper right panel, top), whereas the anti-CHC antibody detected only the CHC in the CCV fraction (lower left panel, top). The AP-3ς subunit was not visible by Coomassie blue staining but was clearly detected by immunoblotting with anti-AP-3ς antibody (upper right panel, bottom). Immunoblotting with anti-AP-1 and anti-AP-2 antibodies demonstrated that these adaptors were undetectable in the purified AP-3 fraction (lower left and right panels). The lower molecular weight bands seen in the CHC and AP-3δ blots most likely represent degradation products of these proteins.