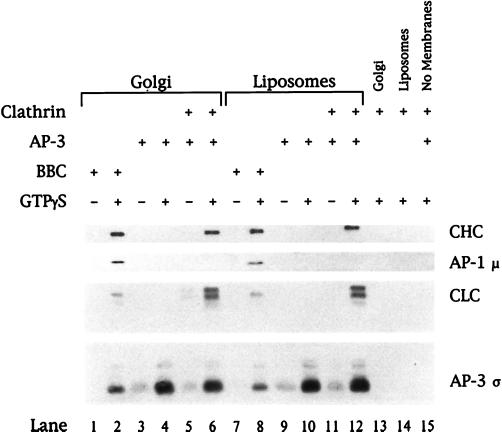
Figure 6.
Recruitment of clathrin onto membranes requires AP-3. Golgi-enriched membranes depleted of AP-1 and ARF (50 μg/ml; lanes 1–6 and 13) or liposomes (200 μg/ml) prepared from soybean 20% PC material (lanes 7–12 and 14) were incubated in reaction mixtures containing either bovine brain cytosol at 5 mg/ml (lanes 1, 2, 7, and 8) or purified AP-3 at 8 μg/ml (lanes 3–6 and 9–12) in the absence or presence of 100 μM GTPγS. Clathrin trimers purified from rat brain CCVs were added to a final concentration of 5 μg/ml (lanes 5, 6, and 11–15). All reactions containing AP-3 and/or clathrin also contained recombinant myristoylated ARF1 (50 μg/ml) and BSA (2.5 mg/ml) to reduce nonspecific protein binding. After recruitment reactions, membranes were recovered and recruited proteins were separated by 12% SDS-PAGE followed by transfer to nitrocellulose. Bound proteins were detected by immunoblotting with a neuron-specific anti-clathrin light chain (CLC) mAb (clone 57.4) or antibodies against the clathrin heavy chain (CHC), AP-1 μ, or AP-3 ς. Species differences between the bovine (lanes 1, 2, 7, and 8) and rat (lanes 5, 6, and 11–15) sources of clathrin are reflected in the migration of the CLCs and the blotting intensity.