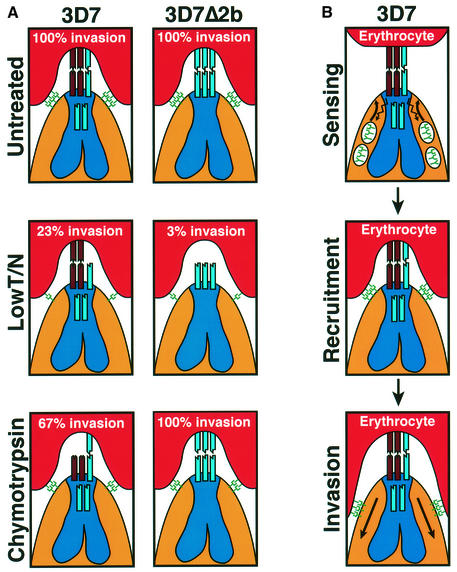
Fig. 6. A simplified model of merozoite invasion into erythrocytes by 3D7 and 3D7Δ2b parasites and the role of the PfRh protein family. (A) A model explaining the enzyme sensitivity of receptor Z as trypsin resistant, neuraminidase resistant and chymotrypsin sensitive. The invasion data are from Table I. Top panels: both 3D7 and 3D7Δ2b parasites show 100% invasion of untreated erythrocytes. The PfRh proteins (shown at the apical end of the merozoites and in the neck of the rhoptries) are responsible for sensing the apical end interaction and signaling to the micronemes for release of high affinity ligands (see B). The eythrocyte-binding antigens (EBAs) and their receptors are shown as green symbols. In 3D7, PfRh2b is responsible for the dominant sensing pathway for invasion via the chymotrypsin-sensitive receptor Z (brown). Disruption of the PfRh2b gene in 3D7 results in replacement of PfRh2b with an alternative pathway specified by another protein [shown as a blue ligand in the neck of the 3D7 rhoptries and interacting with a receptor (blue) on the erythrocyte in 3D7Δ2b] that binds to a chymotrypsin-resistant receptor. Middle panels: following erythrocyte treatment with trypsin and neuraminidase, 3D7 and 3D7Δ2b give 23 and 3% invasion, respectively. The trypsin and neuraminidase resistance of receptor Z is shown. Bottom panels: following erythrocyte treatment with chymotrypsin, 3D7 and 3D7Δ2b give 67 and 100% invasion, respectively. Some invasion can still be achieved using the alternative parasite ligand shown in the rhoptries that binds to a chymotrypsin-resistant receptor. The chymotrypsin sensitivity of receptor Z is shown. (B) Model for the role of the PfRh proteins in sensing the erythrocyte. This would activate release of proteins from micronemes and recruitment at the tight junction, followed by invasion.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.