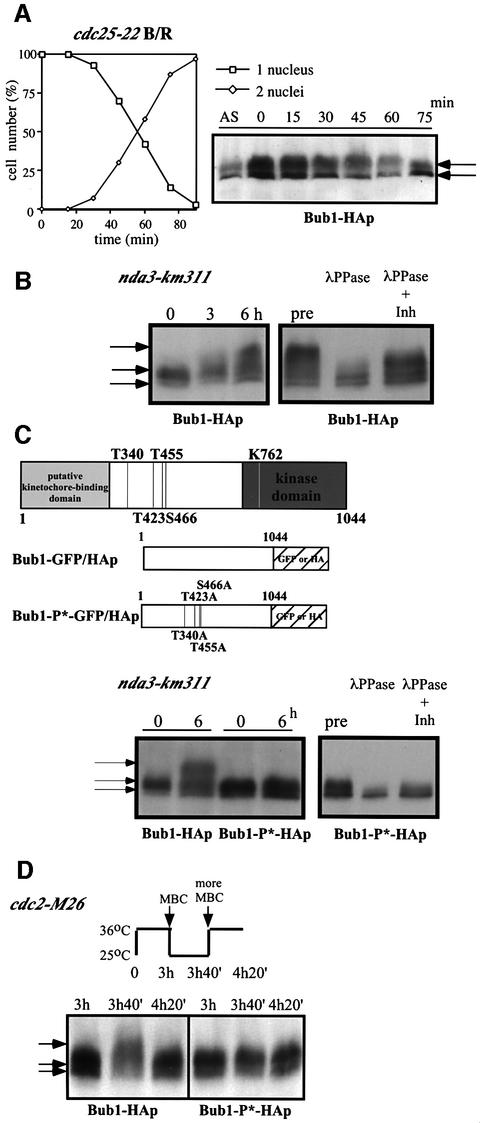
Fig. 1. Phosphorylation of Bub1p during mitosis. (A) Percentage of cells with 1 or 2 nuclei (left panel) and western blotting (right panel) of G2 block and release experiments. Cdc25-22 bub1-HA cells were incubated at 36°C for 4 h, then released at 25°C for the indicated time to proceed through synchronous mitosis. Anti-HA antibody was used to detect Bub1-HAp. (B) Bub1p became hyperphosphorylated in an nda3 block. Nda3-km311 cells were incubated at 18°C for the indicated time in hours (left panel). Extracts were prepared from nda3-km311 cells blocked at 18°C for 6 h (pre) and incubated with λ phosphatase in the absence (λPP) or presence (λPP+Inh) of phosphatase inhibitors (right panel). (C) Hyperphosphorylation was abolished in nda3-km311 bub1-P*-HA strain. The four CDK consensus sites were mutated to alanine in the bub1-P* mutant (left panel). Hyperphosphorylation in an nda3 block was eliminated in bub1-P*-HA strain (centre panel). Phosphatase treatment of extracts from bub1-P*-HA cells (right panel). (D) Hyperphosphorylation of Bub1p was dependent on Cdc2p kinase activity. Cdc2-M26 bub1-HA and cdc2-M26 bub1-P*-HA cells were incubated at 36°C for 3 h, released to 25°C in the presence of 50 µg/ml MBC for 40 min until cells blocked in mitosis, and then shifted back to 36°C in the presence of 100 µg/ml MBC.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.