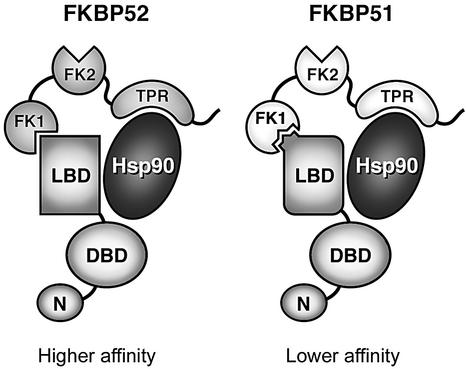
Fig. 8. Model for FKBP-mediated changes in GR hormone-binding affinity. FKBP52 or FKBP51 assemble with GR complexes through an interaction with Hsp90. The three major FKBP domains (FK1, FK2 and TPR) are indicated, as are the major domains of GR (N-terminal domain, DBD and LBD). Hsp90 is a multidomain protein that normally functions as a dimer but, for simplicity, is illustrated as a single entity. When FKBP52 is present in the GR complex, the active PPIase domain (FK1) of FKBP52 interacts with the LBD of GR, stimulating a conformational change and increase in hormone-binding affinity. Conversely, FKBP51 in the GR complex favors an alternative lower-affinity LBD conformation. The distinction between FKBP52 and FKBP51 is perhaps not in PPIase activity per se, but in the specificity of each for sequences within the LBD.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.