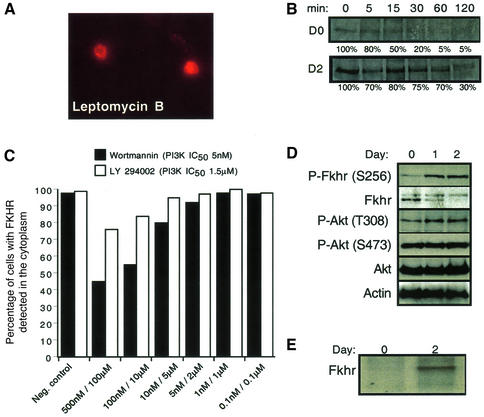
Fig. 4. FKHR shuttling, half-life and phosphorylation are regulated during the differentiation of primary mouse myoblasts. (A) Inhibition of Fkhr nuclear export by Crm1 using leptomycin B (400 nM) in proliferating myoblasts. Cells were incubated for 2 h with leptomycin B, and FKHR was detected by immunofluorescence. (B) The half-life of a His6-tagged FKHR in proliferating (day 0) and differentiating (day 2) myoblasts was determined by immunoprecipitation analyses after a 15 min pulse labeling with [35S]methonine, followed by a 5–120 min chase with cold methionine. (C) Myoblasts treated with the LY294002 (PI3K IC50 = 1.5 µM) inhibitor did not show significant nuclear accumulation of Fkhr even at high doses. However, high doses of wortmannin (100 nM, PI3K IC50 = 5 nM) inhibited 50% of Fkhr nuclear export in growing myoblasts. The effects of the vehicle dimethylsulfoxide (DMSO) are shown as the negative control. (D) Western blot analysis of phosphorylated and non-phosphorylated Fkhr and Akt in proliferating (day 0) and differentiating (day 1 and day 2) myoblasts. Actin was used as a loading control. (E) The phosphorylation status of a His6-tagged FKHR in proliferating (day 0) and differentiating (day 2) myoblasts was determined by immunoprecipitation after a 2 h labeling with [32P]orthophosphate.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.