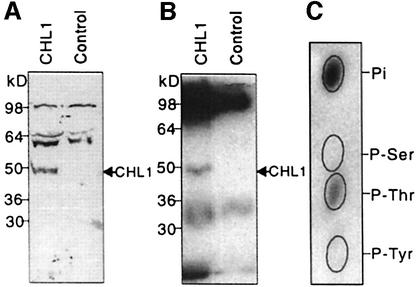
Fig. 2. Phosphorylation of CHL1 at threonine residues. (A) Immunoblot analysis of Xenopus oocyte membrane proteins probed using anti-CHL1 antibodies. Plasma membrane-enriched fractions, isolated from CHL1- or water-injected oocytes (Maurel et al., 1995), were separated by SDS–PAGE for immunoblot analysis using anti-CHL1 antiserum. (B) Autoradiogram of in vitro phosphorylated oocyte membrane proteins immunoprecipitated using anti-CHL1 antibodies. The same fractions used in (A) were phosphorylated in vitro in the presence of [γ-32P]ATP (NEN Life Science Products) and cAMP-dependent kinase catalytic subunit (New England Biolabs). (C) Phosphoamino acid analysis of in vitro phosphorylated CHL1. The 50 kDa phosphorylated peptide on gel (B) was eluted, hydrolyzed and subjected to thin-layer electrophoresis at pH 3.5 (Xu et al., 1996). The ovals show the positions of the non-radioactive standards, phosphoserine (P-Ser), phosphothreonine (P-Thr) and phosphotyrosine (P-Tyr).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.