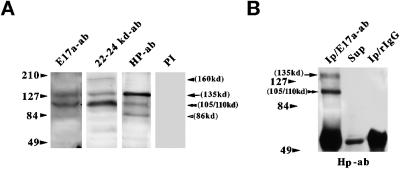
Figure 3.
Characterization of 4.1R isoforms present in skeletal muscle homogenates by Western blotting (A) and immunoprecipitation (B). Three different 4.1R antibodies were used: anti-E17a, anti-22/24 kDa, and anti-Hp; preimmune serum (PI) served as a negative control. In B, the immunoprecipitation assay was performed in the presence of either anti-E17a antibody or control rabbit IgG, as indicated. The blot was subsequently probed with anti-Hp antibody. Sup, supernatant fraction of anti-E17a immunoprecipitate. One-half of the immunoprecipitates (Ip) and one-eighth of the supernatant fraction were loaded onto the respective lanes. The arrows denote the migration position of the ∼135-kDa isoform, and the double arrowheads point to the ∼105/110-kDa protein. The closed and open arrowheads indicate the ∼160- and ∼86-kDa polypeptides, respectively.