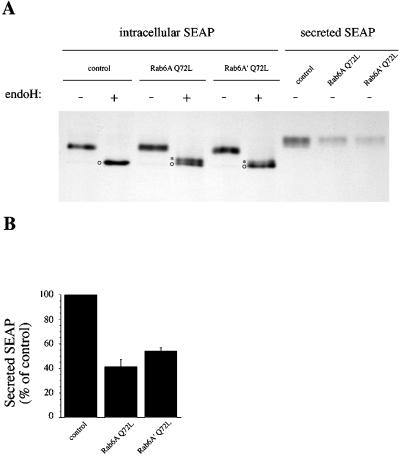
Figure 6.
Both Rab6A Q72L and Rab6A′ Q72L inhibit SEAP secretion and affect N-glycosylation. (A) HeLa cells were cotransfected for 4 h with SEAP plasmids (encoding the SEAP marker) and pGEM-1 (control), Rab6A Q72L–, or Rab6A′ Q72L–encoding plasmids. Cells were then metabolically labeled for 10 min with [35S]methionine and [35S]cysteine and chased for 2 h, and SEAP was immunoprecipitated either from extracellular medium (secreted SEAP) or from the cells (intracellular SEAP). Intracellular SEAP was finally digested (+) or not (−) with endoglycosidase H (endoH) to investigate SEAP processing by Golgi glycosylation enzymes. Asterisks, partially endoH-resistant form; circles, fully endoH-sensitive form. (B) Quantification of secreted SEAP after a 2-h chase (three independent experiments, mean ± SD). Results are expressed as percent of secreted SEAP in control conditions.