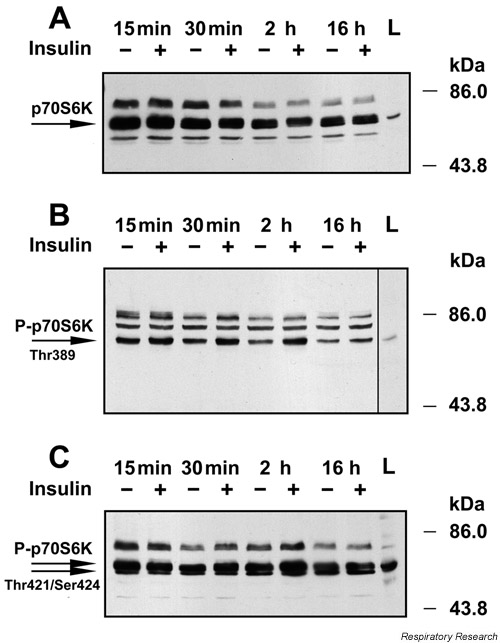
Figure 5.
p70 S6 kinase in H441 cells in the presence or absence of insulin (2.5 μg/ml). Equal amounts of total protein were separated by gel electrophoresis and then probed by immunoblot analysis. Protein molecular weight standards (kDa) are shown on the right. A total lysate of NIH-3T3 cells treated with serum (L) served as a positive control for p70 S6K detection. The data are representative of four experiments. (A) Detection of total p70 S6 kinase. The total amount of p70 S6 kinase declined over time in both the control and insulin-treated conditions with the least amount of protein detected after a 16-hour incubation. There was no effect of insulin on the total amount of p70 S6 kinase when compared to respective control cells at any time point. (B) Detection of phosphorylated p70 S6 kinase. Insulin increased the phosphorylation of Thr389 in p70 S6 kinase at every time point. (C) Detection of phospho-p70 S6 kinase. Insulin increased the phosphorylation of Thr421/Ser424 in p70 S6 kinase after a 30 min and 2 hour incubation time when compared to controls.