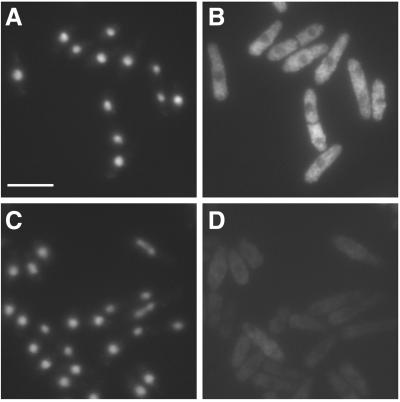
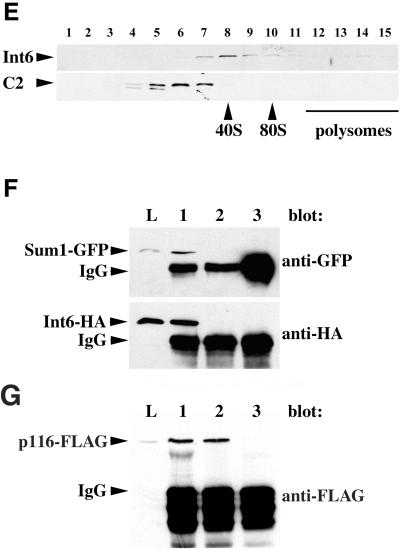
Figure 2.
Fission yeast Int6 has several properties expected of an eIF3 component. (A-D) Indirect immunofluorescence of endogenous Int6. Methanol fixed cells of a wild-type (int6+) strain (A, B) or a strain deleted for int6 (int6Δ, see MATERIALS AND METHODS; (C, D) were processed for immunofluorescence after incubation with affinity-purified anti-Int6 antibodies and mounted in DAPI. For each strain a single representative field was used to collect images of DAPI (DNA) staining (A, C) and Cy3 antirabbit (Int6) staining (B, D). Scale bar, 10 μm. (E) Sucrose density gradient separation of whole cell extracts. After lysis of wild-type (int6+) cells and separation as described in MATERIALS AND METHODS, aliquots of sucrose gradient fractions were subjected to western blotting and were probed with antibodies against Int6 (upper panel) or the proteasome subunit C2 (lower panel). C2 is present in both 20S and 26S proteasomes. The positions at which 40S and 80S ribosomes and polysomes migrated, as determined by monitoring A254 during collection of the fractions, are indicated. (F) Coprecipitation of tagged Int6 and Sum1 proteins. Whole cell lysate (L) or immunoprecipitated proteins from a gene replacement strain expressing Int6-HA and Sum1-GFP (lanes 1 and 3) or a control strain expressing Sum1-GFP and untagged Int6 (lane 2) were subjected to western blotting using antibodies against GFP or HA, as indicated. Immunoprecipitating antibodies were anti-HA (lanes 1,2) or an isotype control (OX34 antirat CD2; lane 3). The immunoprecipitating antibody heavy chain (IgG) was also detected by the HRP-conjugated secondary antibody. (G) Coprecipitation of tagged Int6 and p116 proteins. Whole cell lysate (L) or immunoprecipitated proteins from a strain expressing Int6-HA and p116-FLAG were subjected to western blotting using anti-FLAG antibodies. Immunoprecipitating antibodies were anti-FLAG (lane 1), anti-HA (lane 2) or OX34 (antirat CD2; lane 3).