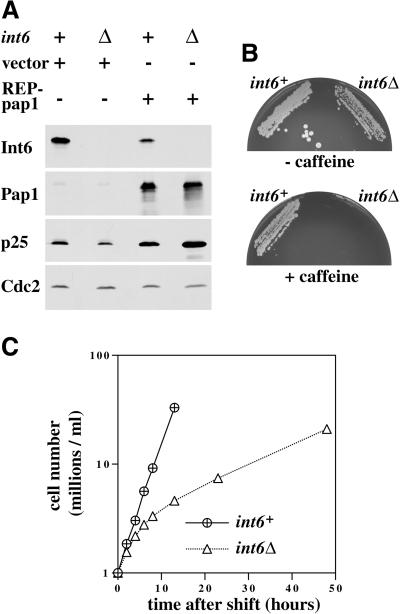
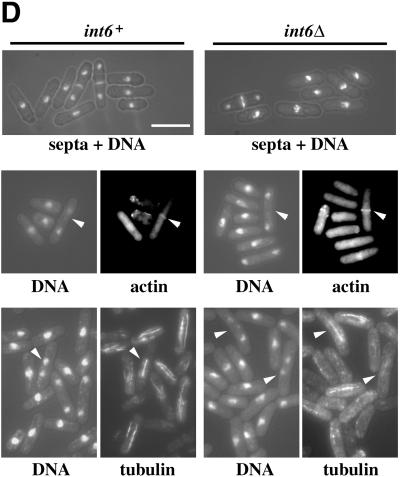
Figure 5.
S. pombe cells lacking Int6 grow slowly, have reduced levels of p25, and are caffeine sensitive. (A) Haploid leu1–32 int6+ (+) and leu1–32 int6::ura4 (Δ) strains transformed either with pREP3X (vector) or pREP3X-pap1 (REP-pap1) were grown in EMM2 at 30°C for 16 h after removal of thiamine. Whole cell extracts prepared from these strains were subjected to Western blotting using antibodies directed against Int6, Pap1, p25, and Cdc2 (loading control), as indicated. (B) Two haploid segregants derived by sporulation of the int6+/int6− strain with genotypes h- int6+ leu1–32 ade- ura4-D18 (int6+) and h- int6::ura4+ leu1–32 ade- ura4-D18 (int6Δ) were streaked onto an EMM2 agar plate supplemented with adenine, leucine, and uracil, either without (upper plate) or with (lower plate) 10 mM caffeine. The plates were photographed after 6 days incubation at 30°C. (C) The int6+ and int6Δ strains were grown to midlog phase in rich (yeast extract) medium before being washed and resuspended in EMM2 medium at 106/ml and grown at 30°C. Cell number was determined for each culture at the times indicated after shifting to EMM2 medium. (D) Samples from each of the cultures used in C were taken at 18 h after shift to EMM2 medium, and were processed for staining with DAPI and calcofluor (visualized simultaneously using UV excitation; upper panels), with DAPI and rhodamine-phalloidin to determine DNA and actin distribution (visualized separately for each of the paired fields shown; middle panels), or with DAPI and an antitubulin antibody (lower panels). Arrowheads indicate cells with actin rings (middle panels) or anaphase cells with mitotic spindles (lower panels). Scale bar, 10 μm.