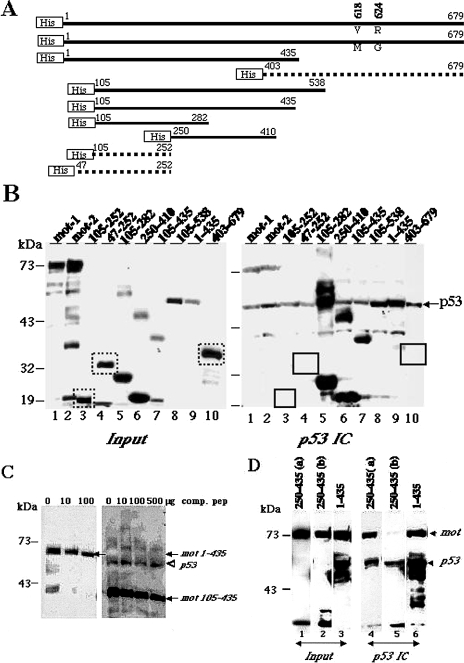
Figure 2.
Mapping the p53 binding domain of the mot. (A) Schematic representation of His-tagged mortalin deletion mutants. The two amino acids (residues 618 and 624) that differ between mot-1 and mot-2 are indicated. The deletion mutants that did not bind to p53 are shown as dotted lines. (B) His-tagged mortalin deletion mutants were added to COS 7 cell lysates and were detected by Western blotting with anti-His tag antibody (input (10%), left panel). p53 immunocomplexes (p53 IC) were analyzed for coprecipitated mortalin by Western blotting with anti-His tag antibody (right panel). The bands corresponding to the deletion mutants that did not show binding are boxed in the input as well as p53 immunocomplexes panels Immunoprecipitated p53 was detected by probing the same membrane with anti-p53 (monoclonal) antibody, indicated by an arrowhead. Extra bands of high molecular weight in lanes 5 and 6 most likely are the aggregated proteins that also coprecipitated with p53. Low-molecular-weight bands in lanes 1, 2, 7, and 10 are the degraded products and did not coprecipitate with p53. (C) Addition of increasing amounts of a peptide (comp. pep) corresponding to a. a. residues 253–282 of mortalin reduced the amount of mot 1–435 a. a. and 105–435 a. a. p53 was immunoprecipitated by a polyclonal anti-p53 antibody (CM-1, Novocastra). Coimmunoprecipitated recombinant mortalin was detected by Western blotting with anti-His tag antibody (indicated by arrows). Immunoprecipitated p53 along with mot 105–435 is shown by an arrow. (D) A high level of expression of mot 250–435 a. a. prevents binding of p53 to endogenous mortalin. COS 7 cells were transfected with expression constructs encoding V5-tagged mortalin deletion mutant proteins spanning residues 250–435 (a — 5 µg DNA and b — 15 µg DNA per 10-cm dish) and 1–435 (15 µgDNA), and the lysates were immunoprecipitated with an anti-p53 antibody (CM-1, Novocastra). p53 immunocomplexes (lanes 4–6) and input protein (lanes 1–3, 10% of the amount of lysate used for immunoprecipitation) were Western blotted with anti-mortalin and anti-V5 tag antibodies to detect endogenous mot (indicated by arrow) and V5-tagged mot fragments, respectively. The p53 immunocomplexes (IC) were also Western blotted with a monoclonal anti-p53 antibody (Pab 421) (indicated by arrowhead) to detect immunoprecipitated p53. Immunoprecipitated p53 and mot 1–435 protein are overlapped in lane 6. Note that only negligible amount of endogenous mot was coprecipitated with p53 from cells that were transfected with a high amount of DNA (lanes 2 and 5).