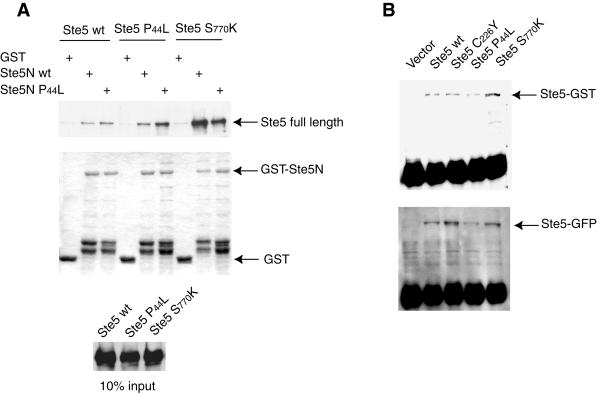
Figure 9.
The P44L and S770K mutations enhance binding of full-length Ste5 to the N terminus of Ste5 without affecting Ste5 oligomerization. (A) GST, or GST-Ste5(1–518) (“Ste5N wt”), or GST-Ste5P44L(1–518) (“Ste5N P44L”), as indicated, were expressed and purified from E. coli, immobilized on glutathione-agarose beads, as described in the legend to Figure 8, and incubated with extracts from ste4Δ ste5Δ cells expressing myc-epitope-tagged versions of full-length wild-type Ste5 (Ste5 wt), Ste5(P44L), or Ste5(S770K), as indicated. After three washes, bound proteins were eluted with 5 mM reduced glutathione, resolved by SDS-PAGE, and analyzed either by immunoblotting with anti-Myc monoclonal antibody 9E10 (top) or by Coomassie blue staining (middle). Samples of the initial extracts (“input”) were also analyzed by immunoblotting with the anti-Myc antibody to demonstrate that Ste5 and the Ste5 mutants were expressed at equivalent levels in these extracts. (B) Either wild-type Ste5 or the indicated Ste5 mutant (each as a GST fusion) was coexpressed with the same form of Ste5 (as a GFP fusion), each expressed from the GAL1 promoter on a plasmid, in ste4Δ ste5Δ cells. Extracts were prepared as described in MATERIALS AND METHODS, and samples (1 mg of total protein) were immunoprecipitated by using polyclonal anti-GST antibodies. Portions of the resulting immunoprecipitates were resolved by SDS-PAGE and examined by immunoblotting with anti-GST antibodies to detect the amount of immunoprecipitated Ste5-GST (top) and with polyclonal anti-GFP antibodies to detect the amount of Ste5-GFP that coimmunoprecipitated (bottom).