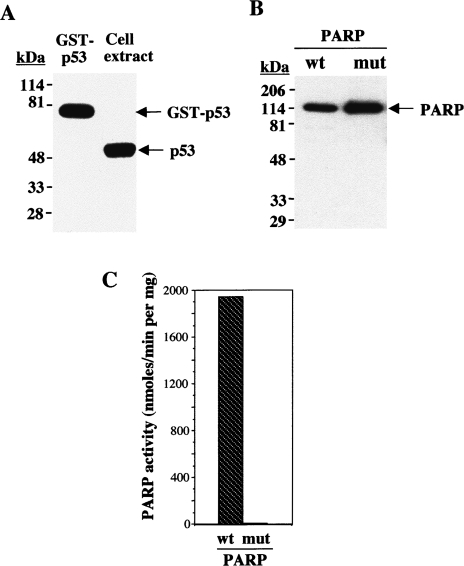
Figure 1.
Immunoblot analysis of recombinant p53 and PARP-1 proteins; catalytic activities of wild-type and mutant PARP-1. (A) Immunoblot analysis with anti-p53 of purified GST-p53(1–393) (0.1 µg) and of an osteosarcoma cell extract (30 µg of protein). (B) Immunoblot analysis with anti-PARP of purified wild-type (wt) and catalytically inactive mutant (mut) PARP-1. The positions of molecular size standards (in kDa) and of the proteins (arrows) are indicated. (C) Relative catalytic activities of wild-type and mutant PARP-1 were determined by incubation of PARP-1 (0.1 µg) for 1 minute at 25°C in a reaction mixture (50 µl) containing 50 mM Tris-HCl (pH 7.8), 25 mM MgCl2, 1 mM dithiothreitol, 4 µg of activated DNA, 100 µM NAD, and 1 µl [32P]NAD (2 mCi/mmol), as described in Materials and Methods section. Data are expressed as nanomoles of [32P]NAD incorporated per minute per milligram of protein, and are means of triplicate determinations from a representative experiment.