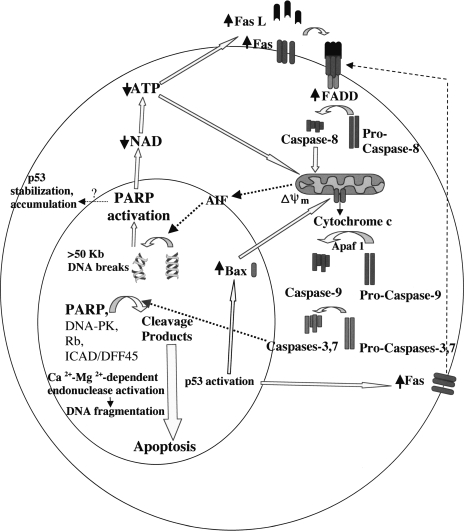
Figure 5.
PARP-1 activation and cleavage in an “amplification loop” toward caspase activation and downstream apoptotic events. Stimulation of the Fas receptor induces the release of mitochondrial factors, such as AIF, that translocates to the nucleus and induces caspase-independent chromatin cleavage into large 50-kb fragments. These large DNA breaks activate PARP, rapidly decreasing NAD and ATP levels, which contributes to both receptor and mitochondrial pathways of apoptosis. Partial depletion of ATP further upregulates Fas, Fas ligand, and FADD; binding of Fas to Fas ligand recruits FADD, resulting in subsequent activation or amplification of the caspase cascade leading to apoptosis. Subsequent cleavage of PARP by caspase-3 prevents depletion of NAD and ATP below a critical level as well as releases certain nuclear proteins, such as Ca2+ -Mg2+-dependent endonucleases and p53, from poly(ADP-ribosyl)ation-induced inhibition.