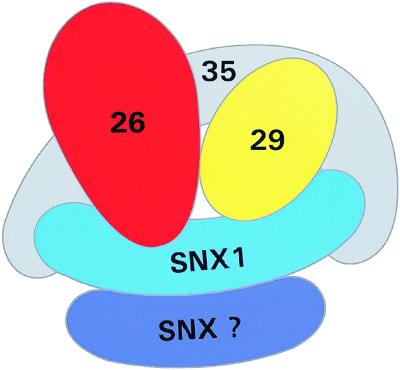
Figure 11.
Model of binding interactions between human retromer proteins. In this model, hVps35 forms the core of a multimeric complex in which it binds directly to at least three other proteins: hVps26, hVps29, and SNX1. Furthermore, there are potential binding interactions that may stabilize the complex: hVps26 with hVps29, hVps29 with SNX1, and SNX1 with SNX1 (or another sorting nexin, designated SNX-?). In contrast, we did not detect evidence for binding of hVps26 directly to SNX 1 or SNX2. Furthermore, SNX2 does not appear to bind directly to hVps35. Although we have depicted a single molecule of SNX1 binding to both binding sites on hVps35, it is possible that each binding site on hVps35 is occupied by distinct molecules of SNX1. Our model for the mammalian retromer complex is consistent with the proposed subunit composition of the yeast retromer complex with the exception that we have not identified a human ortholog for Vps17p (see DISCUSSION).