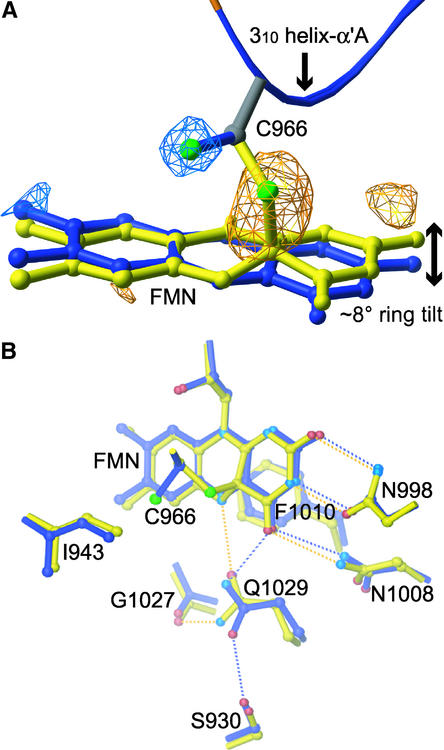
Figure 5.
Conformational Change in the FMN Binding Pocket of Photoexcited LOV2.
(A) Fourfold noncrystallographic symmetry–averaged light-minus-dark difference Fourier map contoured at ±4σ, in which σ is the root-mean-square value of the electron density. Photoactive Cys and the flavin ring for the dark (blue) and photoexcited (yellow) structures are shown. Negative difference density (blue) and positive density (yellow) indicate Cys and ring motion upon photoexcitation.
(B) Side chains exhibiting significant displacements between the dark (blue) and photoexcited (yellow) structures in response to cysteinyl-flavin C(4a) adduct formation. Hydrogen bonds between the protein and FMN cofactor in the dark and photoexcited structures are indicated by blue and yellow dotted lines, respectively. A 2.6 to 3.5 Å range for hydrogen bonding was used. Atoms are colored by elements: nitrogen, light blue; sulfur, green; and oxygen, red. Atoms colored blue in the dark structure and yellow in the photoexcited structure are carbon.