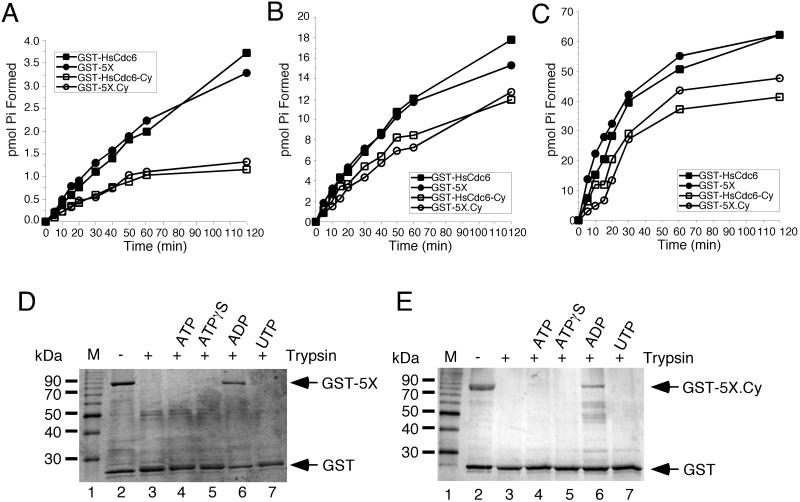
Figure 4.
Nucleotide binding and hydrolysis properties of mutant GST-HsCdc6. (A–C) GST-HsCdc6 (0.25 pmol; ▪), GST-HsCdc6-5X (GST-5X; ●) GST-HsCdc6-Cy (□) and GST-HsCdc6-5X.Cy (GST-5X.Cy; ○) were incubated with (A) 2.5 μM, (B) 25 μM, or (C) 250 μM [γ-32P]ATP for the indicated times at 37°C. Hydrolysis products were separated by TLC, and the amount of phosphate formed was quantified by PhosphorImaging. GST-HsCdc6-5X (0.5 μg; D) or GST-HsCdc6-5X.Cy (E) bound to glutathione agarose was partially digested with trypsin in the absence of nucleotide (lane 3) or in the presence of 2 mM ATP (lane 4), ATPγS (lane 5), ADP (lane 6), or UTP (lane 7). The reaction products were analyzed by 12.5% SDS-PAGE and silver staining. No trypsin was added to the reaction shown in lane 2. M, 10-kDa marker protein ladder.