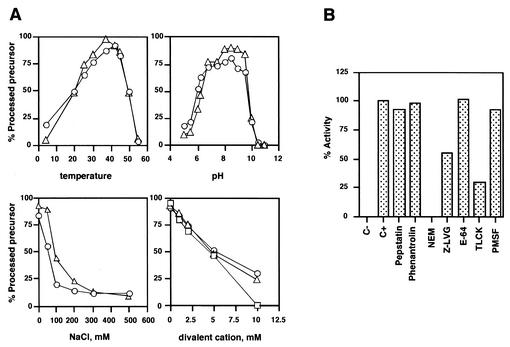
FIG. 3.
(A) pH, temperature, ionic strength, and divalent cation dependence of ASFV protease activity. To study pH dependence, the following buffers were used: 50 mM sodium acetate at pH 5.0, 5.5, and 6.0; 50 mM PIPES at pH 6.2 and 6.8; 50 mM Tris-HCl at pH 7.5, 8.0, and 8.5; 50 mM glycine-NaOH at pH 9.0, 9.5, 10.0, 10.5, and 11.0. The results for the pp62Ct (circles) and pp220frag (triangles) constructs are shown. The following divalent cations were tested with the pp220frag substrate: MgCl2 (triangles), MnCl2 (circles), and CaCl2 (squares). Controls in the presence of pC232S were included for all the conditions assayed. (B) Effect of protease inhibitors on ASFV protease activity. The inhibitors were freshly prepared and added at the following concentrations: pepstatin, 10 μM; phenanthroline, 1 mM; phenylmethylsulfonyl fluoride (PMSF), 1 mM; E-64, 100 μM; Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK), 100 μM; Z-LVG-CHN2, 100 μM; N-ethylmaleimide (NEM), 2.5 mM. They were assayed on full-length pp62 and the pp220 fragment. Activity of 100% corresponded to the processing obtained in the absence of inhibitors for each construct. The data presented are the means of two independent experiments performed on both substrates.