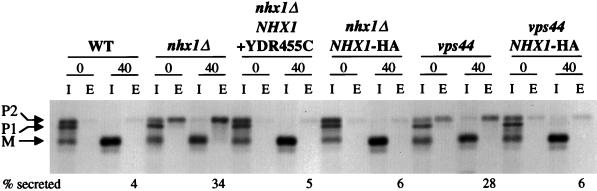
Figure 2.
Secretion of CPY in wild-type (WT), nhx1Δ::Kanr, and vps44 mutant strains, and complementation by NHX1 and NHX1-HA. Cells were labeled with [35S]methionine and cysteine for 10 min, and then chased for 0 or 40 min at 30°C. CPY was immunoprecipitated from intracellular (I) and extracellular (E) fractions for each time point. The positions of ER and Golgi precursor forms of CPY (p1 and p2, respectively), and mature vacuolar CPY (m) are indicated. nhx1Δ cells were transformed with NHX1 and YDR455C on a CEN plasmid (NHX1+YDR455C, pKEB37), or NHX1 alone with an HA epitope tag (NHX1-HA, pKEB38). vps44 cells were transformed with a CEN plasmid containing NHX1 alone with an HA-epitope tag (NHX1-HA, pKEB53). WT and nhx1Δ strains have a wild-type PEP4 gene, encoding proteinase A (required for proteolytic processing). vps44 (vpl27-1) has the pep4-3 mutation, and was transformed with PEP4 on a CEN plasmid (pTS18) for this experiment. The percentage of CPY secreted into the extracellular medium after a 40-min chase was calculated by phosphoimager analysis, and the numbers shown are averages over several separate experiments.