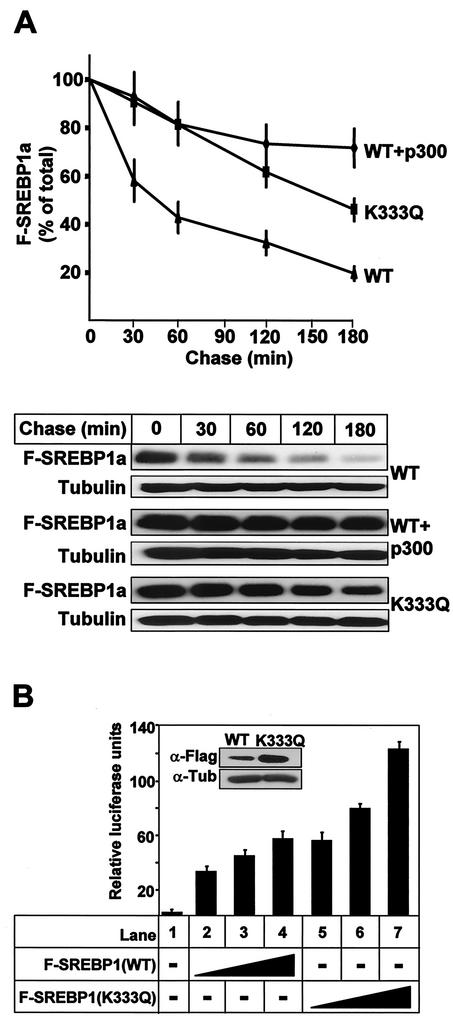
FIG. 7.
Acetylation stabilizes SREBP1a. (A) 293T cells were transfected with Flag-SREBP1a, either the wild type (WT) or the K333Q mutant, in the absence or presence of p300-HA. Thirty-six hours after transfection, cells were treated with cycloheximide for the indicated times, and the levels of Flag-SREBP1a in the cell lysates were determined as described in Materials and Methods. The amount of SREBP1a at each time point is plotted as a percentage of the amount at the start of the chase and represents the average ± the standard error of the mean of three independent experiments (top). The results from one representative experiment are illustrated at the bottom. To ensure that equal amounts of protein were loaded in each well, the levels of tubulin in the samples were estimated by Western blotting using antitubulin antibodies. (B) HepG2 cells were transfected with SYNSRE-luc in the absence (lane 1) or presence (lanes 2 to 7) of increasing amounts of Flag-SREBP1a (1 to 5 ng), either the wild type (lanes 2 to 4) or K333Q (lanes 5 to 7). Thirty-six hours after transfection, the luciferase activity was measured. The data represent the averages ± standard deviations of three independent experiments per-formed in duplicate. The levels of Flag-SREBP1a in samples 4 (WT) and 7 (K333Q) were determined by Western blotting using anti-Flag antibodies (inset).