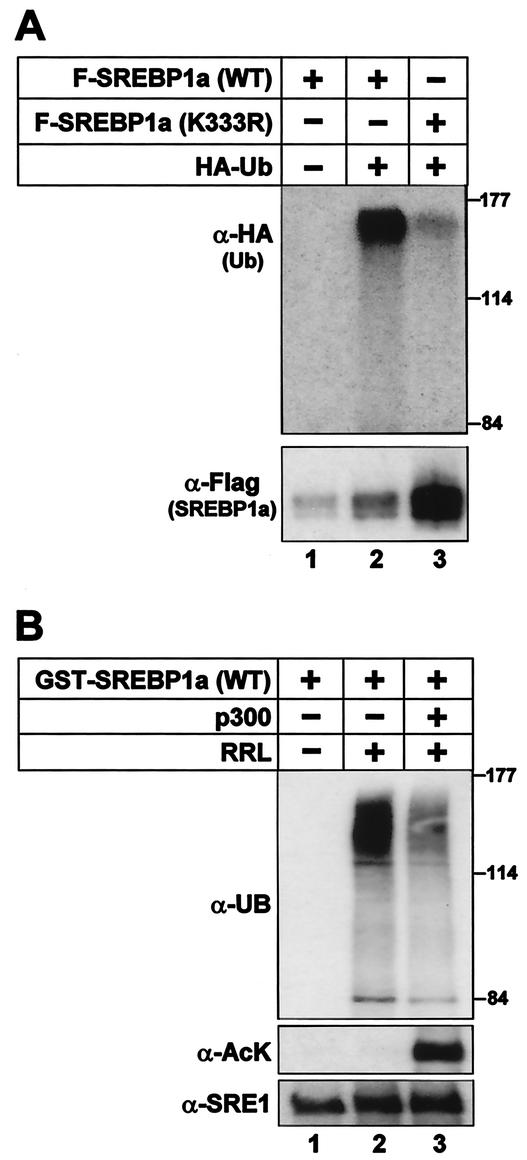
FIG. 8.
Acetylation of SREBP1a prevents its ubiquitination. (A) Flag-tagged wild-type SREBP1a and the K333R mutant were expressed in 293T cells in the absence or presence of HA-ubiquitin. Thirty-six hours after transfection, SREBP1a was immunoprecipitated from cell lysates and resolved by SDS-PAGE. The ubiquitination of SREBP1a was determined by Western blotting using anti-HA antibodies (α-HA). The migration of molecular mass standards (in kilodaltons) is indicated to the right. The levels of SREBP1a in the immunoprecipitates were determined by Western blotting using anti-Flag antibodies (α-Flag). (B) GST-SREBP1a was used as the substrate in in vitro ubiquitination reactions in the absence or presence of rabbit reticulocyte lysate (RRL). Where indicated, GST-SREBP1a was acetylated prior to the ubiquitination reaction (lane 3). The samples were resolved by SDS-PAGE, and the ubiquitination of SREBP1a was determined with antiubiquitin antibodies (α-UB). The migration of molecular mass standards (in kilodaltons) is indicated to the right. The amount of SREBP1a and its acetylation were determined with anti-SREBP1a (α-SRE1) and anti-acetyl lysine (α-AcK) antibodies, respectively.