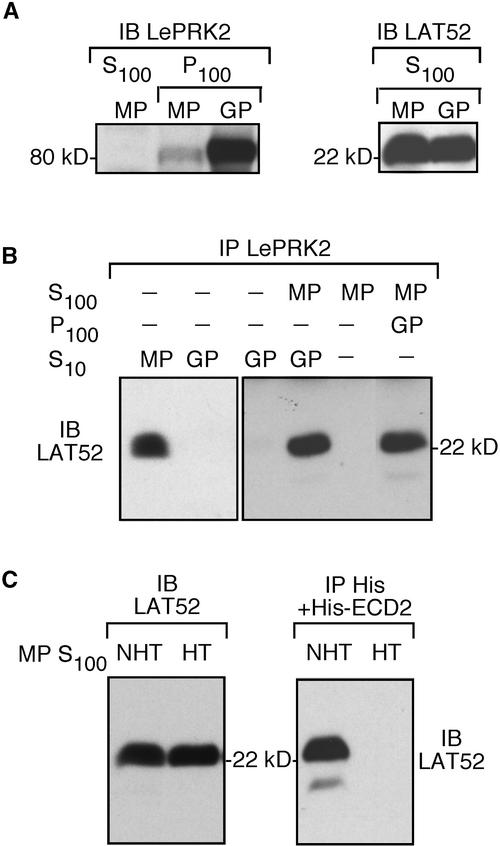
Figure 6.
Under Certain Conditions, LAT52 Cannot Interact with LePRK2.
(A) Detection of LePRK2 (left) and LAT52 (right) by immunoblot (IB) analysis of mature pollen (MP) or germinated pollen (GP) extracts. P100, membrane-associated fraction; S100, soluble fraction.
(B) Coimmunoprecipitation (IP) of LAT52 with LePRK2 from mature pollen extracts, from germinated pollen extracts, or from mixtures, as indicated. At left, mature pollen and pollen germinated in vitro (S10; 1 mg each) were immunoprecipitated with anti-LePRK2 antibody. At right, the germinated pollen extract (S10 or P100, as indicated) was incubated with 500 μg of S100 from mature pollen and immunoprecipitated with anti-LePRK2 antibody. As a control, 500 μg of the S100 from mature pollen also was immunoprecipitated with anti-LePRK2. All immunoprecipitated proteins were subjected to immunoblot analysis with anti-LAT52 antibody. Molecular mass markers are indicated.
(C) Coimmunoprecipitation of heat-treated LAT52 with the extracellular domain of LePRK2. One of two identical aliquots of mature pollen extracts (5 μg/μL) was heated (70°C for 2 min) to obtain a heat-treated (HT) S100, whereas the other aliquot, which was not heat treated (NHT), was held on ice. At left, 2 μL of each aliquot was immunoblotted with anti-LAT52 antibody. At right, the NHT and HT S100 aliquots (200 μL each) were incubated with His-ECD2 protein (5 μg) and precipitated with anti-His antibody. Immunoprecipitated proteins were subjected to immunoblot analysis with anti-LAT52 antibody. Molecular mass markers are indicated.