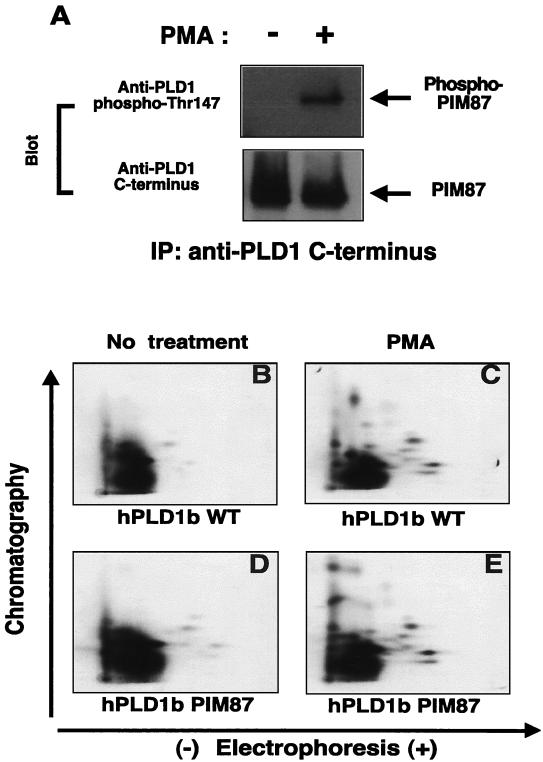
Figure 5.
The PIM87 mutation does not alter PKC-induced phosphorylation of PLD1. (A) After the transfection of PIM87 followed by serum starvation, COS-7 cells were stimulated with 100 nM PMA for 15 min. PIM87 was immunoprecipitated from 2 mg of lysate using an anti-C-terminal PLD1 antibody. The proteins were separated by 8% SDS-PAGE and were subjected to immunoblot analysis using a monoclonal phosphorylation-specific anti-PLD1 antibody or an anti-C-terminal PLD1 antibody. The data are representative of three independent experiments. IP, immunoprecipitating antibody. COS-7 cells were transfected with hPLD1 WT (B, C) or PIM87 (D, E) cDNAs. After the COS-7 cells were loaded with [32P]orthophosphate (0.4 mCi/ml) for 3 h, the cells were stimulated with 100 nM PMA for 15 min. Protein extracts (1.5 mg) then were immunoprecipitated with anti-PLD1 antibody. Immunoprecipitated proteins were separated by 8% SDS-PAGE. The bands of phosphorylated hPLD1 WT and PIM87 were excised and digested with TPCK-trypsin. The tryptic peptides were subjected to two-dimensional phosphopeptide mapping. These data are representative of two independent experiments.