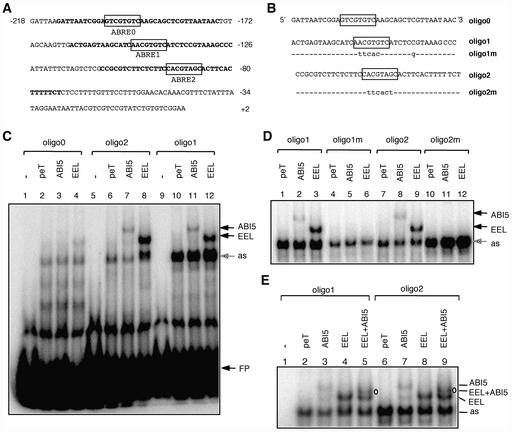
Figure 9.
ABI5 and EEL Interact with the Same ABREs in the AtEm1 Promoter.
(A) Sequence of the minimal AtEm1 promoter. Nucleotides are numbered relative to the transcription start site (+1). ABRE motifs are shown in boxes. Promoter regions used as probes for EMSA are indicated in boldface.
(B) Sequences of the wild-type (oligo0, oligo1, and oligo2) and mutated (oligo1m and oligo2m) oligonucleotides used as probes for EMSA. Mutated nucleotides are indicated in lowercase letters. Sequences are presented in the 5′ to 3′ orientation and are shown as single strand only.
(C) ABI5 and EEL bind to oligo1 and oligo2. Oligo0 (lanes 1 to 4), oligo2 (lanes 5 to 8), and oligo1 (lanes 9 to 12) were incubated without protein extract (free probe; lanes 1, 5, and 9) or with in vitro–transcribed and translated vector pET16b (negative control; lanes 2, 6, and 10), ABI5 (lanes 3, 7, and 11), or EEL (lanes 4, 8, and 12). Bands shifted by ABI5 or EEL are indicated by arrows. as, an aspecific band shifted by reticulocyte proteins; FP, free probe.
(D) Mutations in ABRE1 and ABRE2 abolish the binding of ABI5 and EEL. Oligo1 (lanes 1 to 3), oligo1m (lanes 4 to 6), oligo2 (lanes 7 to 9), or oligo2m (lanes 10 to 12) were incubated with in vitro–transcribed and translated vector pET16b (negative control; lanes 1, 4, 7, and 10), ABI5 (lanes 2, 5, 8, and 11), or EEL (lanes 3, 6, 9, and 12). In contrast to the wild-type oligo1 and oligo2, the mutant oligo1m and oligo2m were not shifted by ABI5 and EEL proteins.
(E) ABI5 and EEL bind to oligo1 and oligo2 as homodimers and as a heterodimer. Oligo1 (lanes 1 to 5) or oligo2 (lanes 6 to 9) were incubated without protein extract (free probe; lane 1) or with in vitro–transcribed and translated vector pET16b (negative control; lanes 2 and 6), ABI5 (lanes 3 and 7), EEL (lanes 4 and 8), or cotranslated ABI5 and EEL (lanes 5 and 9). The shifted band corresponding to the ABI5-EEL heterodimer is indicated (EEL + ABI5).