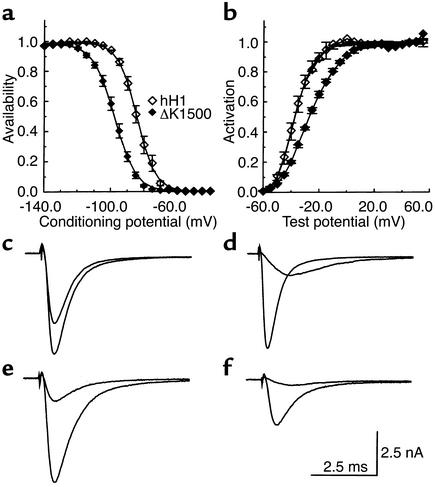
Figure 3.
Shifts in gating kinetics associated with the ΔΚ1500 mutant. (a) Na+ channel availability was determined with 500-ms pulses to various potentials followed by test pulses to –20 mV. Test pulse currents were normalized and plotted against the conditioning potential. Gating parameters were obtained from fits with Boltzmann functions. V1/2h was –84.5 ± 1.3 mV (n = 9) for the wild-type and –97.9 ± 1 mV (n = 13) for the ΔΚ1500 mutant; the slope factor was 4.8 ± 0.1 and 6.2 ± 0.1 mV, respectively. (b) Activation currents were obtained with test pulses from a holding potential of –100 mV. V1/2h was –47.5 ± 1.5 mV for the wild-type and 49.5 ± 0.3 mV for the ΔΚ1500; slope factor was 7.5 ± 0.4 and 13 ± 0.3 mV, respectively. (c–f) Representative current recordings from cells expressing the wild-type (c and d) and ΔΚ1500 mutant channel (e and f). (c and e) Current obtained at a test potential of –20 mV after conditioning depolarizations to –140 and –90 mV. (d and f) Currents obtained at test potentials of –40 and –20 mV from a holding potential of –100 mV.