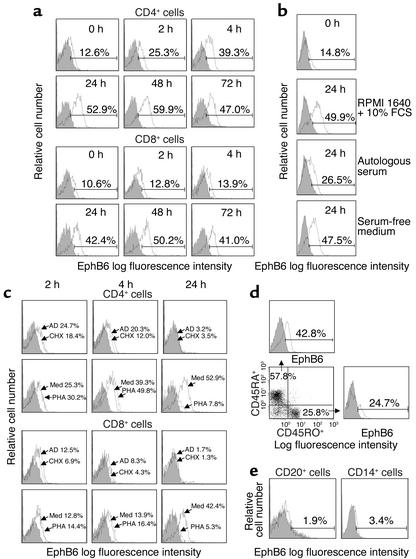
Figure 1.
Flow cytometry analysis of EphB6 expression on immune cells. (a) MACS-purified CD4+ or CD8+ T cells (more than 98% pure) were cultured for different times and analyzed for EphB6 expression by two-color flow cytometry using FITC-labeled anti-CD4 or FITC-labeled anti-CD8 in combination with biotin-conjugated anti-EphB6 and PE-streptavidin. Histograms show EphB6 expression on cells gated on CD4+ or CD8+ populations. Percentages represent EphB6+ populations in the gated regions after deducting background staining (isotypic Ab, shaded area); this is also the case for all other histograms in Figure 1. (b) MACS-purified T cells were cultured in regular RPMI 1640 medium, 100% fresh serum from the blood donor (autologous serum), or serum-free medium for 24 hours. EphB6 expression was examined by one-color flow cytometry. (c) MACS-purified CD4+ or CD8+ cells were cultured in the presence of PHA (1 μg/ml), actinomycin D (AD; 4 μg/ml), cycloheximide (CHX; 20 μg/ml), or in medium (Med) alone for the indicated time periods. Cells were stained and analyzed as described in a. (d) MACS-purified T cells were cultured for 24 hours and then stained with Quantum Red–labeled anti-CD45RO, FITC-labeled anti-CD45RA, and biotin-conjugated anti-EphB6 followed by PE-streptavidin. The CD45RO+ and CD45RA+ cells were separately gated, and their EphB6 expression was assessed. (e) The T cell–depleted fraction of PBMCs was stained with FITC-labeled anti-CD20 or FITC-labeled anti-CD14 in combination with biotin-conjugated anti-EphB6 and PE-streptavidin, and analyzed by two-color flow cytometry. EphB6 expression on cells gated on CD20+ or CD14+ populations is shown.