Abstract
We have induced in canines long-term immune tolerance to an allogeneic cell line derived from a spontaneous canine astrocytoma. Allogeneic astrocytoma cells were implanted endoscopically into the subcutaneous space of fetal dogs before the onset of immune competency (<40th gestational day). At adulthood, dogs rendered tolerant successfully serve as recipients of intracranial transplants of their growing allogeneic, subcutaneous tumor. Transplanted dogs subsequently develop a solid brain tumor with histological features similar to the original astrocytoma. This model may allow rapid development and evaluation of new therapies for brain tumors, as well as afford tumor biology studies that are untenable in smaller, immune incompetent, or inbred animals harboring less representative tumors.
Keywords: glioma, animal model, immune tolerance, astrocytoma
Introduction
Progress in development and testing new anticancer therapies is impeded or delayed by the lack of models accurately manifesting features of spontaneously arising neoplasms. For primary brain tumors, the low incidence in humans [1,2], coupled with the precipitous lethality of this cancer [3,4], leads to a small and inhomogeneous population by tumor location, patient age, and histological characteristics of malignant astrocytoma [5,6]. Because surgery is the single most effective treatment for patients with a brain tumor [7–9], an optimal brain tumor model for testing new treatments would use an animal of sufficient size to allow meaningful surgical resection of the tumor. Additionally, the tumor should manifest histological, immunological, genetic, and therapeutic determinants analogous to those of the spontaneous human counterpart [10].
Canine brain tumors very closely approximate the human disease relative to histopathology, epidemiology, and clinical course [11–16]. Such lesions have proven useful in development of imaging studies of intracranial masses [17,18], as well as in pioneering novel radiation treatment strategies [19–21]. A spontaneous canine anaplastic astrocytoma was developed into a long-term cell line, DL3580c2 [22], which grows anchorage-independently, over-expresses epidermal growth factor receptor, and is tumorigenic in athymic mice. No mutations in exons 4, 5, 6, 7, and 8 of the canine p53 gene [23–25] have been detected in this cell line.
DL3580c2 cells were used to induce allogeneic immune tolerance in outbred beagles according to paradigms and hypotheses exploring this concept [26–30]. It is believed that appropriately timed (fetal or neonatal) exposure to allogeneic cells can induce native immune tolerance. Whereas functional immune tolerance can be induced in experimental animals by the creation of hematopoietic-chimeric animals [31], the intent here was to preserve the constitutive immunological identity of the host while inducing tolerance to allogeneic cells. We decided to deposit the allogeneic astrocytoma cells into the subcutaneous space of fetal dogs because of the rich immune exposure of this tissue, and the immunologically naive or preimmune status of fetal pups [32].
Materials and Methods
Gestational ages of time-dated beagles (Marshall Farms, NY) were determined by serial ultrasound measurements of chorionic sac diameter, crown-rump length, and head diameter [33]. Fifty-microliter implants containing 107 DL3580c2 cells were delivered separately to each fetus via endoscope into the subcutaneous space within the flank region. Briefly, on the 37th gestational day, the gravid uterus was exposed through a midline abdominal incision. A self-retaining introducer fitted with a Teflon seal was installed into the uterus through a 4-mm incision. Access to the fetus was gained by use of a 2.8-mm rigid lens endoscope (Karl Storz Endoscopy-America, Inc., Culver City, CA) with a 1.0-mm working channel; illumination was provided with a 150 W tungsten halogen lamp. Cells were implanted through a 30-cm TFE 30TW cannula (Cole-Parmer Instrument Co.) fitted with a 26-gauge sharp needle.
Fetal and placental membranes are potentially significant obstructions to clear viewing of, and access to, the fetal skin. The magnitude of the obstruction is highly dependent on fetal age; at later gestational ages (>42 days) the membranes retract tightly around the fetus, becoming less entangling to endoscopic approaches. Scheduling the fetal implants before the 40th gestational day was based on reports that later dates were outside the tolerance-induction window for soluble antigens in dogs [32].
Results
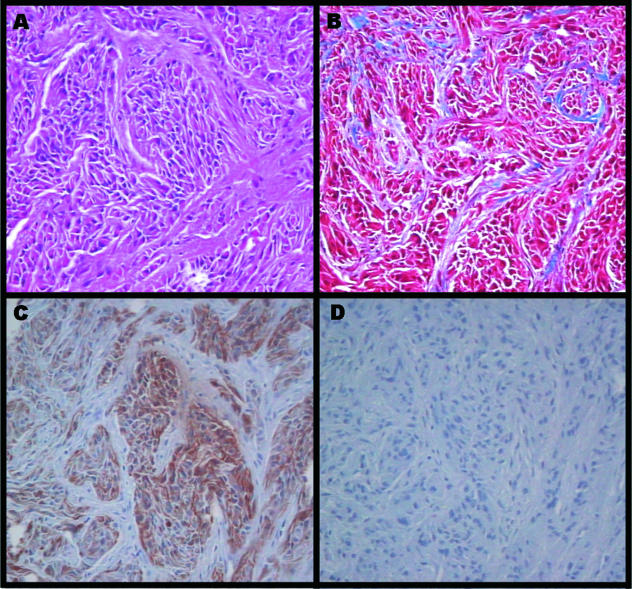
Within the first 6 weeks after birth, pups may develop palpable masses at the site of fetal implantation. After 5 months the lesions were resected and processed for histological analysis. Conventional hematoxylin & eosin (H & E) staining showed tightly packed bundles of tumor cells enmeshed in a rich connective tissue network (Figure 1A). Trichrome staining showed the presence of collagenous fibers around the tumor bundles (Figure 1B). Immunohistochemical staining for glial fibrillary acidic protein (GFAP) showed strong expression of this glial cell-specific intermediate filament marker (Figure 1C). Comparative karyotypic analysis confirmed that the subcutaneous tumor was derived from the cells implanted during fetal development (data not shown).
Figure 1.
Histological view of subcutaneous tumor generated from subcutaneous allogeneic astrocytoma implant into fetal dogs. H&E stained section (A) shows bundles of fibrillary malignant cells within a network of connective tissue. Trichrome staining (B) shows a rich collagenous network of fibers around the tumor cell bundles. Immunostaining with anti-GFAP antibodies (DAKO Corp., Carpinteria, CA). (C) portrays a strong presence of this glial-specific intermediate filament protein. (D) The isotype matched negative section. (Original magnification, 40 x objective).
During the same single surgical procedure under isofluorane anesthesia, regions of the resected subcutaneous tumors (glial lineage confirmed on cryostat sections) were mechanically dissociated into a fresh cell brie, and 50 µL were injected over a 2-minute interval intracranially into the left internal capsule by using stereotactic coordinates [34] and an external frame (David Kopf Instruments, Tujunga, CA). Hydrostasis in the cranium after the injection was re-established by using bone wax in the burr hole, and the scalp incision was closed in a conventional fashion. After recovery from anesthesia, the animals were returned to routine kennel stay including circadian photoperiods, daily play intervals and ad libitum dog chow according to IACUC-approved protocol.
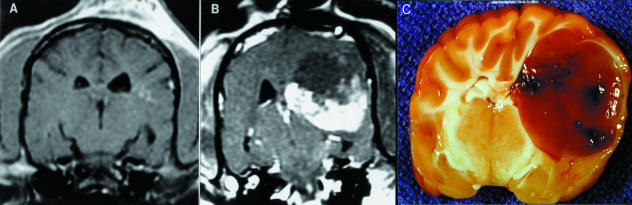
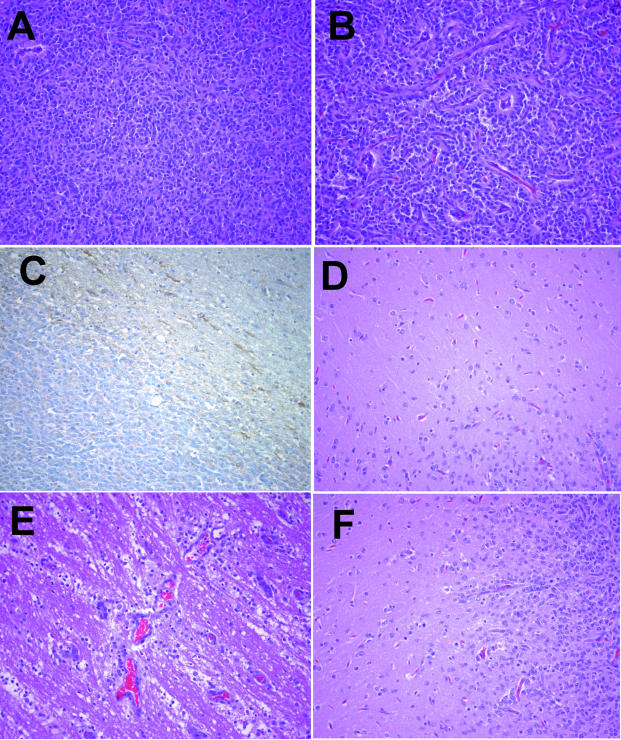
Tumor growth was followed by cranial magnetic resonance imaging (MRI) studies every 5 to 7 weeks. MRI of the brain was performed by using T1-, intermediate, and T2-weighted images. The intermediate and T2-weighted images were performed by using a fast-spin echo, dual-echo technique with a TR 300/TE 40/80 ms on a GE Signa (General Electric, Milwaukee, WI). After the intravenous infusion of paramagnetic contrast material (gadolinium-DTPA, Magnevist Abbott Laboratories, Abbott Park, IL), T1-weighted images were collected in the axial and sagittal planes. Five weeks after intracranial transplantation, MRI showed no definitive diagnostic aberrations (Figure 2A). Seven weeks later, a large, anatomically displacing, hemorrhagic mass was evident (Figure 2B). At necropsy, a gross cranial coronal section showed a highly vascular, apparently well-demarcated tumor (Figure 2C). On H&E stained sections, the microscopic features included regions of dense hypercellularity with mitotic figures (Figure 3A, pseudopalisading necrosis, and rich neovascularization (Figure 3B). These are the criteria for a diagnosis of World Health Organization (WHO) grade IV astrocytoma (glioblastoma multiforme) [35–37]. Immunohistochemical studies indicated weak staining by anti-GFAP antibodies (Figure 3C). Modulation of GFAP expression in response to environmental signals, cell density, and malignant transformation has been reported [38–40].
Figure 2.
Clinical and postmortem appearance of transplanted intracranial astrocytoma. T1-weighted MRI images after injection of paramagnetic contrast agent 5 weeks (A) and 10 weeks (B) after intracranial implant. Coronal section of the brain at necropsy confirmed the MRI findings, showing a large, anatomically displacing, hemorrhagic lesion in the left parietal lobe with extension of the mass and edema into the left frontal and temporal lobes.
Figure 3.
Histological view of intracranial allogeneic astrocytoma. Regions of the brain specimen shown in Figure 2 were processed in formalin and sectioned from paraffin blocks. The tumor showed heterogeneous hyperdense regions of proliferative, malignant cells with pleomorphic nuclei (A); surrounding areas showed regions of neovascularization (B). The intracranial tumor only stained weakly for GFAP (C). Although grossly the tumor appeared to be well demarcated, there were regions of single astrocytoma cell percolation into white matter (D), conduits of invasion along perivascular structures (E), and wide areas of generalized astrocytoma invasion into adjoining normal brain (F). (Original magnification, 20x objective).
Because local invasion is such a clinical problem in management of patients with astrocytomas [41–43], brain sections adjacent to the tumor were processed to determine patterns of the allogeneic astrocytoma invasion. Solitary tumor cell infiltration into white matter (Figure 3D), perivascular trajectories (Figure 3E), as well as star-burst-like invasion from the rim of the tumor (Figure 3F) were identified. Overall, the tumor showed the typical centrally expansive and peripherally diffusively infiltrative growth that characterizes high grade astrocytomas.
Of 13 attempts to induce allogeneic tolerance by subcutaneous implants into fetal pups (wherein a litter of pups was successfully whelped), seven litters harbored dogs who eventually developed glial tumors. The most successful procedure was a litter wherein 4 of 5 litter mates sustained allogeneic tumor growth. Reasons for failure may include inadequate allogeneic cell inoculum [27], arrested or stunted immune tolerance due to tardiness of the implant [28], or possible incompatible major immunohistocompatibility genotypes between allograft and host [44–46]).
Discussion
Successful modeling of astrocytoma in a large animal that preserves significant pathologic features of the spontaneous disease is likely to afford new opportunities for accelerated and novel therapy development [10,47,48]. Whereas an intracranial xenograft of human gliomas in cats has been reported [49], this system relies on aggressive and persistent immunosuppression of the host, which renders the model unsuitable for immune-based therapies. Even treatments relying on genetic manipulation of the tumor, such as gene therapy, may require a functioning immune system to accurately evaluate the consequences of the intervention [35,50–52]. A transplantable canine brain tumor model in immune competent hosts has been used over the past decade [16,53], but this tumor shows a growth pattern and histological features more consistent with a gliosarcoma.
The brain tumor model described in this report demonstrates the propagation of a spontaneous, allogeneic canine astrocytoma in immune competent dogs. The tumor properties match well the growth patterns, histological characteristics, and molecular pathology of human astrocytomas. Because of the surgically accessible anatomical size of the model and the intact immune status of the host, this allogeneic astrocytoma model in dogs may serve as an effective tool for accelerated cancer therapy development and testing. These may include gene therapy [54], radiation treatment [55], novel treatment delivery strategies such as enhanced convection delivery [56–58] or slow release formulations [59,60] in a system appropriate for prehuman studies. Because novel cancer treatments inevitably must pass toxicity testing in canines [54], it may be advantageous to use the allogeneic astrocytoma model to optimize treatment delivery schemes before human trials.
Acknowledgements
Dr. Kim H. Manwaring at the Phoenix Children's Hospital provided critical expertise and access to endoscopic instrumentation. Drs. Paul Keller and Burton Drayer at Barrow Neurological Institute generated and interpreted the MRI films. Patty Gesswein assisted in the surgical procedures. Technical expertise in cell culture and fetal implants was provided by Monique D. Rief, Melinda A. Loo, Wendy Mc-Donough and Sherri Treasurywala. The project was funded by a grant from the Barrow Neurological Foundation. Persistent encouragement and support from Sr. Nancy Perlick, OFM, is gratefully acknowledged. Procedures for “Induction of immune tolerance to tumor cells” are protected under U.S. Patent #5,723,718.
References
- 1.Berens ME, Rutka JT, Rosenblum ML. Brain tumor epidemiology, growth and invasion. Neurosurg Clin N Am. 1990;1:1–18. [PubMed] [Google Scholar]
- 2.Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG. The descriptive epidemiology of brain and central nervous system tumors in the United States. Results from CBTRUS 1990–1994. Neuro-Oncology. 1999;1:14–25. doi: 10.1093/neuonc/1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin VA, Gutin PH, Leibel S. Neoplasms of the Central Nervous System. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. 4th ed. Philadelphia, PA: J.B. Lippincott; 1993. pp. 1679–1746. [Google Scholar]
- 4.Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: An analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J Neurosurg. 1998;88:1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Winger MJ, Macdonald DR, Schold SC, Cairncross JG. Selection bias in clinical trials of anaplastic glioma. Ann Neurol. 1989;26:531–534. doi: 10.1002/ana.410260406. [DOI] [PubMed] [Google Scholar]
- 6.Kirby S, Brothers M, Irish W, Florell R, Macdonald D, Schold C, Cairncross G. Evaluating glioma therapies: Modeling treatments and predicting outcomes. J Natl Cancer Inst. 1995;87:1884–1888. doi: 10.1093/jnci/87.24.1884. [DOI] [PubMed] [Google Scholar]
- 7.Wisoff JH, Boyett JM, Berger MS, Brant C, Li H, Yates AJ, McGuire-Cullen P, Turski PA, Sutton LN, Allen JC, Packer RJ, Finlay JL. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: A report of the Children's Cancer Group trial no. CCG-945. J Neurosurg. 1998;89:52–59. doi: 10.3171/jns.1998.89.1.0052. [DOI] [PubMed] [Google Scholar]
- 8.Vecht CJ, Avezaat CJ, van Putten WL, Eijkenboom WM, Stefanko SZ. The influence of the extent of surgery on the neurological function and survival in malignant glioma. A retrospective analysis in 243 patients. J Neurol Neurosurg Psychiatry. 1990;53:466–471. doi: 10.1136/jnnp.53.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander E, Loeffler JS. Radiosurgery for primary malignant brain tumors. Semin Surg Oncol. 1998;14:43–52. doi: 10.1002/(sici)1098-2388(199801/02)14:1<43::aid-ssu6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Knapp DW, Waters DJ. Naturally occurring cancer in pet dogs: important models for developing improved cancer therapy for humans. Mol Med Today. 1997;3:8–11. doi: 10.1016/s1357-4310(96)20031-0. [DOI] [PubMed] [Google Scholar]
- 11.Palmer AC. Comparative aspects of tumors of the central nervous system in the dog. Proc Royal Soc Med. 1976;69:49–51. [PMC free article] [PubMed] [Google Scholar]
- 12.Bigner DD, Swenberg JA. Experimental Tumors of the Central Nervous System. Kalamazoo, MI: The Upjohn Company; 1977. First English edition translated from W. Janisch and D. Schreiber. [Google Scholar]
- 13.Rubinstein LJ. Correlation of animal brain tumor models with human neurooncology. NCI Monograph. 1977;46:43–49. [PubMed] [Google Scholar]
- 14.Frenier SL, Moore MP, Kraft SL, Gavin PR. Canine intracranial astrocytomas and comparison with the human counterpart. The Small Animal Compendium. 1990;12:1422–1433. [Google Scholar]
- 15.Heidner GL, Kornegay JN, Page RL, Dodge RK, Thrail DE. Analysis of survival in a retrospective study of 86 dogs with brain tumors. J Vet Intern Med. 1991;5:219–226. doi: 10.1111/j.1939-1676.1991.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 16.Zaki FA. Spontaneous central nervous system tumors in the dog. Vet Clin North Am. 1977;7:153–163. doi: 10.1016/s0091-0279(77)50013-5. [DOI] [PubMed] [Google Scholar]
- 17.Turrel JM, Fike JR, LeCouteur RA, Higgins RJ. Computed tomographic characteristics of primary brain tumors in 50 dogs. J Am Vet Med Assoc. 1986;188:851–856. [PubMed] [Google Scholar]
- 18.Kraft SL, Gavin PR, DeHaan C, Moore M, Wendling LR, Leathers CW. Retrospective review of 50 canine intracranial tumors evaluated by magnetic resonance imaging. J Vet Intern Med. 1997;11:218–225. doi: 10.1111/j.1939-1676.1997.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 19.Gavin PR, Kraft SL, DeHaan CE, Swartz CD, Griebenow ML. Large animal normal tissue tolerance with boron neutron capture. Int J Radiat Oncol Biol Phys. 1994;28:1099–1106. doi: 10.1016/0360-3016(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 20.Gavin PR, Kraft SL, Huiskamp R, Coderre JA. A review: CNS effects and normal tissue tolerance in dogs. J Neurooncol. 1997;33:71–80. doi: 10.1023/a:1005773331737. [DOI] [PubMed] [Google Scholar]
- 21.Whelan HT, Schmidt MH, Segura AD, McAuliffe TL, Bajic DM, Murray KJ, Moulder JE, Strother DR, Thomas JP, Meyer GA. The role of photodynamic therapy in posterior fossa brain tumors. A preclinical study in a canine glioma model. J Neurosurg. 1993;79:562–568. doi: 10.3171/jns.1993.79.4.0562. [DOI] [PubMed] [Google Scholar]
- 22.Berens ME, Bjotvedt G, Levesque DC, Rief MD, Shapiro JR, Coons SW. Tumorigenic, invasive, karyotypic and immunological characterization of clonal cell lines derived from a spontaneous canine anaplastic astrocytoma. In Vitro Cell Dev Biol Anim. 1993;29A:310–318. doi: 10.1007/BF02633959. [DOI] [PubMed] [Google Scholar]
- 23.Devilee P, Van Leeuwen IS, Voesten A, Rutteman GR, Vos JH, Cornelisse CJ. The canine p53 gene is subject to somatic mutations in thyroid carcinoma. Anticancer Res. 1994;14:2039–2046. [PubMed] [Google Scholar]
- 24.Van Leeuwen IS, Hellmen E, Cornelisse CJ, Van den Burgh B, Rutteman GR. P53 mutations in mammary tumor cell lines and corresponding tumor tissues in the dog. Anticancer Res. 1996;16:3737–3744. [PubMed] [Google Scholar]
- 25.Veldhoen N, Milner J. Isolation of canine p53 cDNA and detailed characterization of the full length canine p53 protein. Oncogene. 1998;16:1077–1084. doi: 10.1038/sj.onc.1201863. [DOI] [PubMed] [Google Scholar]
- 26.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 27.Ramsdell F, Fowlkes BJ. Maintenance of in vivo tolerance by persistence of antigen. Science. 1992;257:1130–1134. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- 28.Fadem BH, Hill HZ, Huselton CA, Hill GJ. Transplantation, growth, and regression of mouse melanoma xenografts in neonatal marsupials. Cancer Invest. 1988;6:403–408. doi: 10.3109/07357908809080068. [DOI] [PubMed] [Google Scholar]
- 29.Edwards RG, Jauniaux E, Binns RM, Layton M, Jurkovic D, Grillo TA, Campbell S. Induced tolerance and chimaerism in human fetuses using coelocentesis: A medical opportunity to avert genetic disease? Hum Reprod Update. 1995;1:419–427. doi: 10.1093/humupd/1.4.419. [DOI] [PubMed] [Google Scholar]
- 30.Alferink J, Tafuri A, Vestweber D, Hallmann R, Hammerling GJ, Arnold B. Control of neonatal tolerance to tissue antigens by peripheral T cell trafficking. Science. 1998;282:1338–1341. doi: 10.1126/science.282.5392.1338. [DOI] [PubMed] [Google Scholar]
- 31.Kim HB, Shaaban AF, Yang EY, Liechty KW, Flake AW. Microchimerism and tolerance after in utero bone marrow transplantation in mice. J Surg Res. 1998;77:1–5. doi: 10.1006/jsre.1997.5255. [DOI] [PubMed] [Google Scholar]
- 32.Tizard I. Veterinary Immunology: An Introduction. 4th ed. Philadelphia, PA: WB Saunders Co; 1992. [Google Scholar]
- 33.Yeager AE, Mohammed HO, Meyers-Wallen V, Vannerson L, Concannon PW. Ultrasonographic appearance of the uterus, placenta, fetus, and fetal membranes throughout accurately timed pregnancy in Beagles. Am J Vet Res. 1992;53:342–351. [PubMed] [Google Scholar]
- 34.Lim RK, Liu C, Moffitt RL. A Stereotaxic Atlas of the Dog's Brain. Springfield, MO: Charles C. Thomas; 1960. [Google Scholar]
- 35.Sobol RE, Fakhrai H, Shawler D, Gjerset R, Dorigo O, Carson C, Khaleghi T, Koziol J, Shiftan TA, Royston I. Interleukin-2 gene therapy in a patient with glioblastoma. Gene Ther. 1995;2:164–167. [PubMed] [Google Scholar]
- 36.Ojeda VJ, Moutaery KR. Neuropathological biopsy diagnoses in clinical practice: How thorough and reproducible are they? Pathology. 1998;30:328–329. doi: 10.1080/00313029800169576. [DOI] [PubMed] [Google Scholar]
- 37.Schiffer D. Classification and biology of astrocytic gliomas. Forum (Genova) 1998;8:244–255. [PubMed] [Google Scholar]
- 38.Herpers MJ, Budka H, McCormick D. Production of glial fibrillary acidic protein (GFAP) by neoplastic cells: adaptation to the microenvironment. Acta Neuropathol (Berl) 1984;64:333–338. doi: 10.1007/BF00690398. [DOI] [PubMed] [Google Scholar]
- 39.Gomes D, de Nechaud B, Maunoury R, Moura Neto V, Brigaudeau C, Labrousse F, Dupouey P. Glial fibrillary acidic protein expression in a new human glioma cell line in culture before and after xenogenic transplantation into nude mice. Acta Neuropathol (Berl) 1997;94:376–384. doi: 10.1007/s004010050722. [DOI] [PubMed] [Google Scholar]
- 40.Pekny M, Eliasson C, Chien CL, Kindblom LG, Liem R, Hamberger A, Betsholtz C. GFAP-deficient astrocytes are capable of stellation in vitro when cocultured with neurons and exhibit a reduced amount of intermediate filaments and an increased cell saturation density. Exp Cell Res. 1998;239:332–343. doi: 10.1006/excr.1997.3922. [DOI] [PubMed] [Google Scholar]
- 41.Scherer HJ. The forms of growth in gliomas and their practical significance. Brain. 1940;63:1–35. [Google Scholar]
- 42.Laerum OD, Bjerkvig R, Steinsvag SK, de Ridder L. Invasiveness of primary brain tumors. Cancer Metastasis Rev. 1984;3:223–236. doi: 10.1007/BF00048386. [DOI] [PubMed] [Google Scholar]
- 43.Laws ER, Jr, Goldberg WJ, Bernstein JJ. Migration of human malignant astrocytoma cells in the mammalian brain: Scherer revisited. Int J Devel Neurosci. 1993;11:691–697. doi: 10.1016/0736-5748(93)90056-j. [DOI] [PubMed] [Google Scholar]
- 44.Thomson AW, Lu L, Murase N, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells (Dayt) 1995;13:622–639. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz P, Nassiri M, Viciana AL, Padmanabhan J, Streilein JW. Characterization of donor chimerism, alloreactive host T cells and memory cell development in thymi from mice resistant to neonatal transplantation tolerance. J Immunol. 1995;154:633–643. [PubMed] [Google Scholar]
- 46.Ruiz P, Nassiri M, Gregorian S, Viciana AL, Streilein JW. Neonatal transplantation tolerance is associated with a systemic reduction in memory cells, altered chimeric cell phenotype, and modified eicosanoid and cytokine production. Transplantation. 1996;61:1198–1205. doi: 10.1097/00007890-199604270-00014. [DOI] [PubMed] [Google Scholar]
- 47.Crafts D, Wilson CB. Animal models for brain tumors. Natl Cancer Inst Monograph. 1977;46:11–17. [PubMed] [Google Scholar]
- 48.Peterson DL, Sheridan PJ, Brown WE. Animal models for brain tumors: historical perspectives and future directions. J Neurosurg. 1994;80:865–876. doi: 10.3171/jns.1994.80.5.0865. [DOI] [PubMed] [Google Scholar]
- 49.Krushelnycky BW, Farr-Jones MA, Mielke B, McKean JD, Weir BK, Petruk KC. Development of a large-animal human brain tumor xenograft model in immunosuppressed cats. Cancer Res. 1991;51:2430–2437. [PubMed] [Google Scholar]
- 50.Andreansky S, He B, van Cott J, McGhee J, Markert JM, Gillespie GY, Roizman B, Whitley RJ. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998;5:121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 51.Herrlinger U, Kramm CM, Johnston KM, Louis DN, Finkelstein D, Reznikoff G, Dranoff G, Breakefield XO, Yu JS. Vaccination for experimental gliomas using GM-CSF-transduced glioma cells. Cancer Gene Ther. 1997;4:345–352. [PubMed] [Google Scholar]
- 52.Tseng SH, Hwang LH, Lin SM. Induction of antitumor immunity by intracerebrally implanted rat C6 glioma cells genetically engineered to secrete cytokines. J Immunother. 1997;20:334–342. doi: 10.1097/00002371-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Salcman M, Scott EW, Schepp RS, Knipp HC, Broadwell RD. Transplantable canine glioma model for use in experimental neuro-oncology. Neurosurgery. 1982;11:372–381. doi: 10.1227/00006123-198209000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Guidance for Industry, author. S6 Preclinical Safety Evaluation Of Biotechnology-Derived Pharmaceuticals. Rockville, MD: Drug Information Branch (HFD-210), Center for Drug Evaluation and Research (CDER); 1997. pp. 1–14. [Google Scholar]
- 55.Williams JA, Dillehay LE, Tabassi K, Sipos E, Fahlman C, Brem H. Implantable biodegradable polymers for IUdR radiosensitization of experimental human malignant glioma. J Neurooncol. 1997;32:181–192. doi: 10.1023/a:1005704913330. [DOI] [PubMed] [Google Scholar]
- 56.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kroll RA, Pagel MA, Muldoon LL, Roman-Goldstein S, Neuwelt EA. Increasing volume of distribution to the brain with interstitial infusion: dose, rather than convection, might be the most important factor. Neurosurgery. 1996;38:746–752. [PubMed] [Google Scholar]
- 58.Groothuis DR, Ward S, Itskovich AC, Dobrescu C, Allen CV, Dills C, Levy RM. Comparison of 14C-sucrose delivery to the brain by intravenous, intraventricular, and convection-enhanced intracerebral infusion. J Neurosurg. 1999;90:321–331. doi: 10.3171/jns.1999.90.2.0321. [DOI] [PubMed] [Google Scholar]
- 59.Fung LK, Ewend MG, Sills A, Sipos EP, Thompson R, Watts M, Colvin OM, Brem H, Saltzman WM. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998;58:672–684. [PubMed] [Google Scholar]
- 60.Garcia-Contreras L, Abu-Izza K, Lu DR. Biodegradable cisplatin microspheres for direct brain injection: preparation and characterization. Pharm Dev Technol. 1997;2:53–65. doi: 10.3109/10837459709022609. [DOI] [PubMed] [Google Scholar]