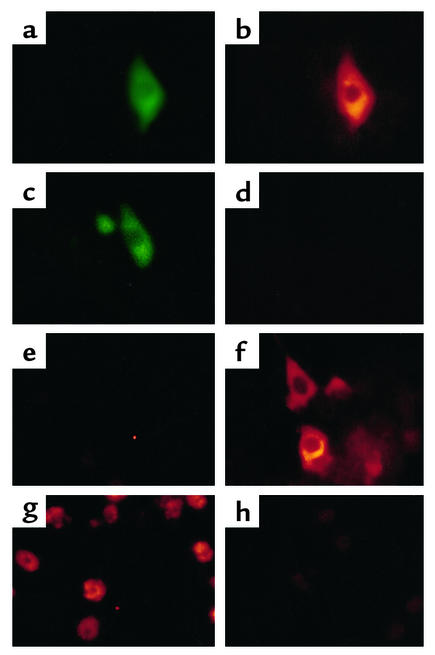
Figure 4.
HCV protein synthesis in infected primary Tupaia hepatocytes. (a–d) Immunofluorescence of transfected primary Tupaia hepatocytes. To assess specificity and sensitivity of anti-HCV antibodies for immunofluorescence, primary Tupaia hepatocytes were cotransfected with a plasmid (pCDHCV.S1b) expressing the HCV structural proteins, and with a GFP-expression construct (pEGFP-N1) (a and b), or with pEGFP-N1 and pcDNA3 (c and d). Forty-eight hours after transfection, the cells were fixed with a 1:1 mixture of methanol/acetone (vol/vol) and incubated with a well-characterized serum panel (24, 25) from HCV-infected individuals containing high-titer anti-HCV antibody (diluted 1:100 in PBS with 1% BSA), followed by Cy3-conjugated anti–human IgG secondary antibody (b and d). To identify and locate transfected individual cells, the cells were coincubated with anti-GFP antibody, followed by an FITC-conjugated anti–rabbit IgG antibody. (a and c). (e–h) Immunofluorescence of infected Tupaia hepatocytes. For HCV infection, primary Tupaia hepatocytes were incubated with HCV RNA–positive serum 1 day after plating. On days 1 (e) and 5 (f and g) after infection, viral protein expression was analyzed by immunofluorescence using anti–HCV-specific antibodies as described above. Staining was positive in 1–5% of Tupaia hepatocytes (f and g). Anti-HCV immunoreactivity (g) could be inhibited by incubation of anti-HCV antibodies with recombinant HCV proteins prior to use in immunofluorescence (h). Magnification: ×40 (a–f) and ×10 (g and h).