Figure 1.
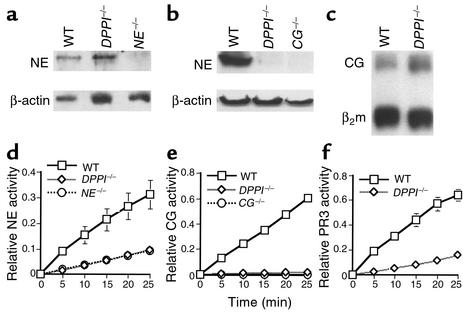
Analysis of PMN-derived serine proteases in DPPI–/– mice. NE (a) and CG (b) protein expression in DPPI–/– bone marrow lysates. β-actin served as control for protein content. Note the markedly reduced level of immunoreactive CG in DPPI–/– bone marrow lysate compared with WT. (c) S1 nuclease protection assay revealed equivalent levels of CG mRNA in DPPI–/– and WT bone marrow lysate. β2 microglobulin (β2m) served as control for RNA loading. (d) DPPI–/– bone marrow cells and NE–/– bone marrow cells have equivalent residual levels of NE activity. (e) Both DPPI–/– and CG–/– bone marrow cells have no detectable hydrolysis of the CG-specific peptide substrate. (f) Conversion of the PR3 substrate by DPPI–/– bone marrow cell lysate was reduced by 80% compared with WT. Relative enzyme activity was measured at OD405 per 105 bone marrow cells and values represent mean activity ± SEM of at least three animals.