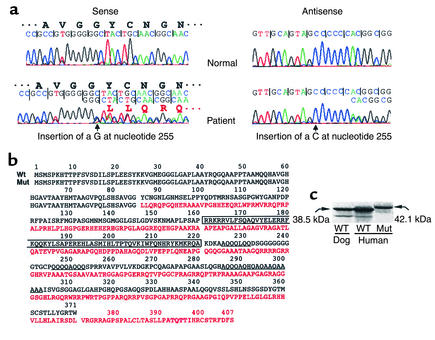
Figure 1.
Identification of the mutation in the TTF1 gene and synthesis of the mutTTF1. (a) Fragments of sequence chromatograms of PCR-amplified genomic DNA from a normal individual and the patient. Sense and the corresponding antisense sequences show the insertion of a G and a C, respectively (arrows), at position 255 of the patient’s TTF1 gene (numbering beginning with the first coding nucleotide in exon 2). The mutation involves one of the two alleles producing a “double” sequence beyond the insertion site. The encoded amino acids of wild-type and mutant TTF1 genes are indicated. (b) The deduced amino acid sequence of the mutTTF1 (divergent sequence in red) compared with the wtTTF1 (in black). The homeobox and the glutamine/alanine–rich regions of the wtTTF1 are boxed and underlined, respectively. Note that these sequences are lost in the mutTTF1, which has 36 additional amino acids. (c) The products of in vitro translation, analyzed by SDS-PAGE. One microgram of plasmid DNA containing the wild-type (dog and human) TTF1 and mutTTF1 cDNAs served as template in a coupled transcription/translation reaction in rabbit reticulocyte lysate in the presence of 35S-Met. The dried gel was then radioautographed. The larger size of the mutTTF1 was confirmed. A smaller amount of a lower–molecular weight product represents a product translated from a downstream ATG, as previously described (13). Molecular weights were deduced from the amino acid sequences. Wt, wild-type.