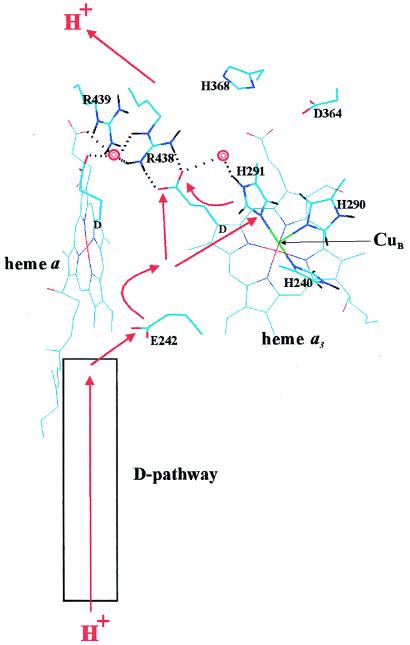
Figure 1.
Proton transfer in the heme–copper oxidases. The view is approximately in the membrane plane with the proton input and output sides of the membrane below and above, respectively. The figure is based on the x-ray coordinates of cytochrome aa3 from Paracoccus denitrificans (ref. 13; Protein Data Bank identification code 1AR1), drawn by using the program HyperChem 4.5 (Hypercube, Ontario, Canada). The D rings of hemes a and a3 are marked and the D-propionate groups are highlighted. CuB in the foreground (green), and some key residues are both highlighted and marked. Two crystallographically visible water molecules (13) are shown as red double rings. Dotted lines indicate potential hydrogen bonds discussed in the text. The red arrows outline possible paths of pumped protons across the enzyme’s membrane domain (see text).