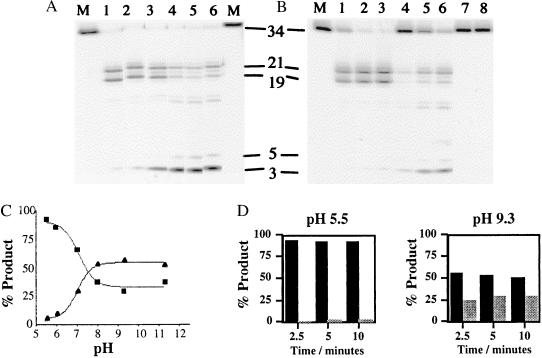
Figure 1.
The product distribution of T5 exonuclease reaction varies with pH. (A) Activity was assayed on the pseudo-Y substrate, with 10 mM MgCl2 cofactor. Lanes marked M show the result of reactions in mixtures that contained no enzyme, and lanes 1–6 contained 4 nM wild-type enzyme in buffers at pH 5.5, 6.0, 7.0, 8.0, 9.3, and 11.3, respectively. The sizes (nucleotides) of the products are as indicated. (B) Time courses of wild-type 5′-3′ exonuclease at pH extremes. Wild-type exonuclease, at a concentration of 0.8 nM, was incubated with the labeled pseudo-Y substrate at 37°C for 2.5, 5, or 10 min at pH 5.5 (lanes 1–3) or pH 9.3 (lanes 4–6) in the presence of 10 mM MgCl2. The products of the reaction were separated on a 7 M urea/15% acrylamide gel. Untreated substrate, lane M, and substrates incubated at 37°C for 10 min at each pH in the absence of enzyme (lanes 7 and 8) are shown. (C) Phosphoimager data from A showing the percentage of product plotted against pH for both exonucleolytic (▴) and endonucleolytic (■) cleavage. (D) Graphical representation of the time course at pH 5.5 and 9.3 from data obtained from B. Endonucleolytic product is shown by dark bars, exonucleolytic product, by light bars. Differences between C and D in the absolute levels of exo- and endonuclease activity reflect the concentration of the enzyme used. In C all of the original substrate has been degraded.