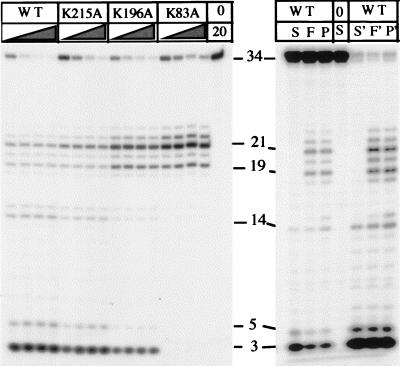
Figure 3.
Degradation of pseudo-Y substrate by T5 5′-3′ exonuclease lysine mutants. (Left) Enzymes were incubated with the substrate at 37°C for 2.5, 5, 10, or 20 min in the presence of 10 mM MgCl2, and reaction products were separated by denaturing PAGE. The concentration of the K83A and K196A mutants (40 nM) was 10 times higher in the reaction than that of the K215A mutant and the wild-type enzyme (4 nM). A control reaction lacking enzyme was incubated for 20 min (lane 20). (Right) Wild-type enzyme (20 nM) was incubated with pseudo-Y substrate (P), single-stranded oligonucleotide (S), or a full flap structure (F). Reactions were sampled at 2 and 30 min (first and last three lanes, respectively).