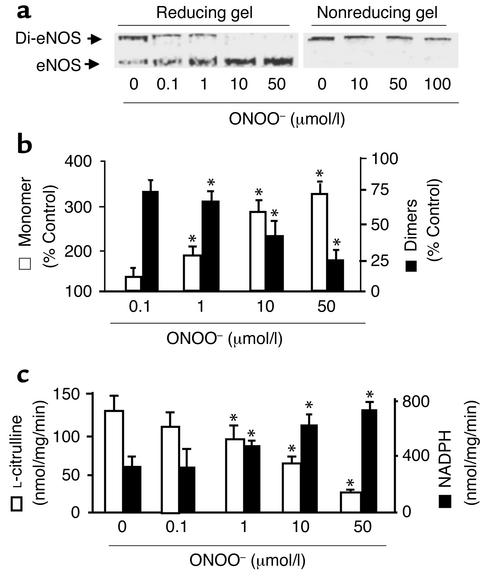
Figure 1.
ONOO– dissociates SDS-resistant dimers and alters recombinant eNOS activity. Purified eNOS was treated with ONOO– (0–100 μmol/l), decomposed ONOO–, or NaOH vehicle (100 mmol/l) as described in Methods. Five minutes after treatment, eNOS dimers and monomers were separated by low-temperature SDS-PAGE (6%) under reducing or nonreducing conditions. The proteins were visualized by Coomassie staining. (a) Representative blots of eNOS dimers and monomers in reducing (left) or nonreducing gels (right). (b) ONOO– dissociated eNOS dimers into monomers under reducing conditions. The intensity (area times density) of dimers and monomers was determined by densitometry as described in Methods. The results were expressed as percent change compared with untreated enzyme (n = 10, *P < 0.05). (c) Inhibition by ONOO– (0.1–50 μmol/l) of the rate of eNOS-dependent L-citrulline formation (n = 12) was associated with an increase in NADPH oxidation (n = 14). L-citrulline formation and NADPH oxidation by purified recombinant eNOS were each assayed as described in Methods. *P < 0.01. Di-eNOS, eNOS dimer; eNOS, eNOS monomer.