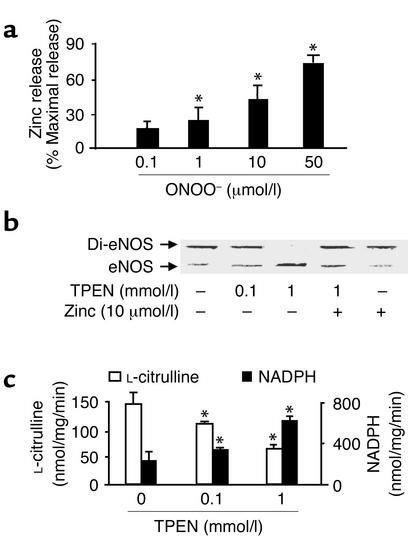
Figure 2.
Monomer formation and activity of recombinant eNOS associated with zinc release. (a) ONOO– (0.1 – 50 μmol/l) stimulated zinc release from purified eNOS (n = 9, *P < 0.01). Zinc was assayed as described in Methods and was expressed as percentage of maximal zinc release from eNOS diluted in 7 mol/l guanidine HCl. (b) Dissociation of eNOS dimers by zinc chelator TPEN. Purified eNOS was exposed to TPEN (0.1 or 1 mmol/l) or methanol (control) in the presence or absence of exogenous zinc at room temperature for 30 minutes in 0.1 M HEPES buffer, pH 7.5. eNOS protein was subjected to low-temperature SDS-PAGE under reducing conditions, and the protein was visualized by Coomassie staining. Blot shown is representative of four independent experiments. (c) The effect of TPEN on eNOS function. The activity of eNOS treated with TPEN was assayed as described in Methods. The figure represents results obtained in six independent assays (n = 6, *P < 0.05).