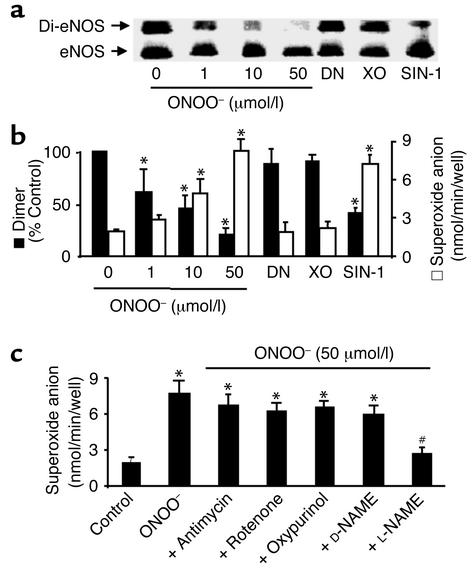
Figure 4.
ONOO– dissociates eNOS dimers and triggers O2.– release in cultured BAECs. (a) ONOO– (1–50 μmol/l) increased dissociation of eNOS dimers in intact BAECs. The cells were treated with ONOO– as described in Methods, and eNOS dimers and monomers were separated by low-temperature SDS-PAGE under reducing conditions. The eNOS protein was blotted onto nitrocellulose membranes and detected with a monoclonal antibody as described in Methods. Blot shown is representative of six independent experiments. (b) ONOO– and SIN-1 decreased eNOS dimer but increased O2– release in intact BAECs. Confluent BAECs were treated with ONOO– (50 mmol/I), SIN-1 (5 mmol/l), DEA-NONOate (DN; 5 mmol/l), or O2.– generated from 10 mU/ml xanthine oxidase in the presence of 0.1 mmol/l hypoxanthine (XO). The cells were rinsed twice with 2 ml PBS buffer (pH 7.4) and then exposed to calcium ionophore A23187 (10 μmol/l) for 2 hours. The amount of A23187-stimulated O2.– was measured by the SOD-inhibitable cytochrome c reduction assay as described in Methods (n = 6 or 8, *P < 0.05). (c) eNOS-dependent O2.– release in BAECs treated with ONOO–. Antimycin (10 μmol/l), rotenone (10 μmol/l), oxypurinol (10 μmol/l), D-NAME (1 mmol/l), or L-NAME (1 mmol/l) was added 10 minutes after treating BAECs with ONOO– and 10 minutes before the addition of A23187 (n = 10; *P < 0.05 compared with untreated cells). The increase in O2.– measured in cells exposed to ONOO– was prevented by L-NAME (1 mmol/l; n = 8, #P < 0.01).