Abstract
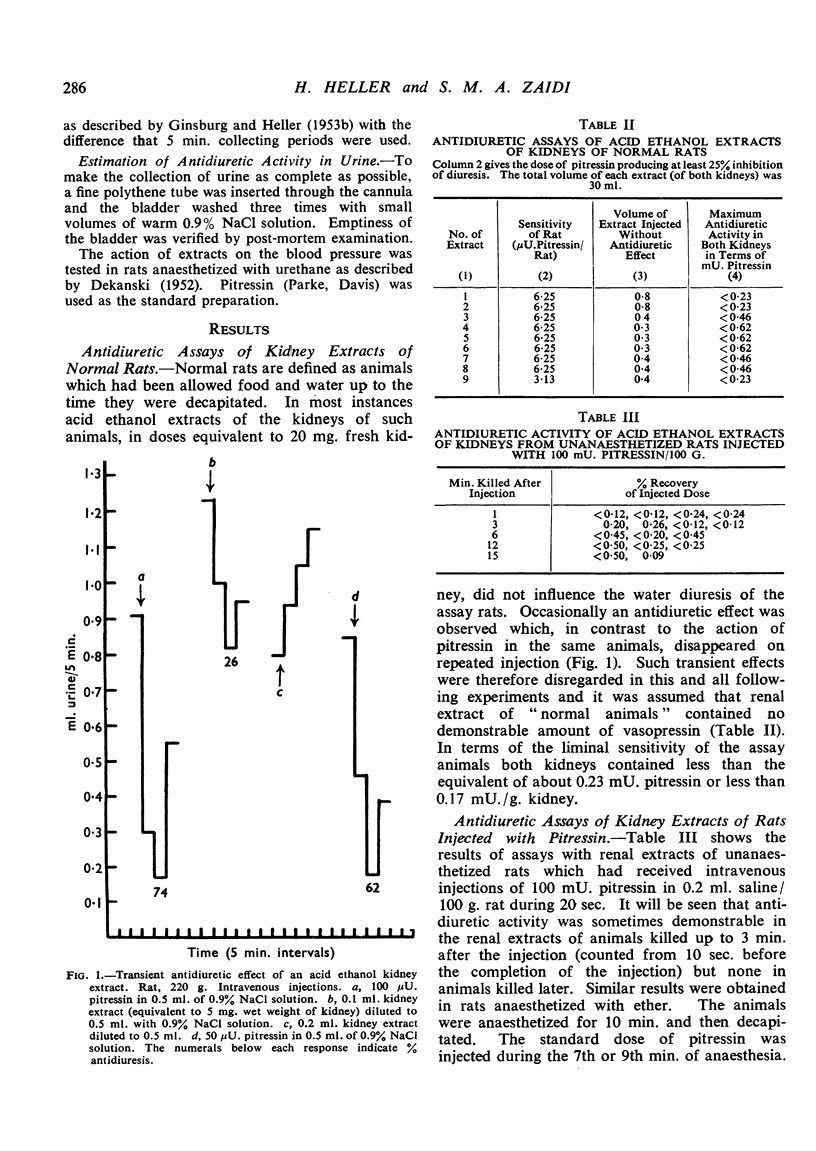
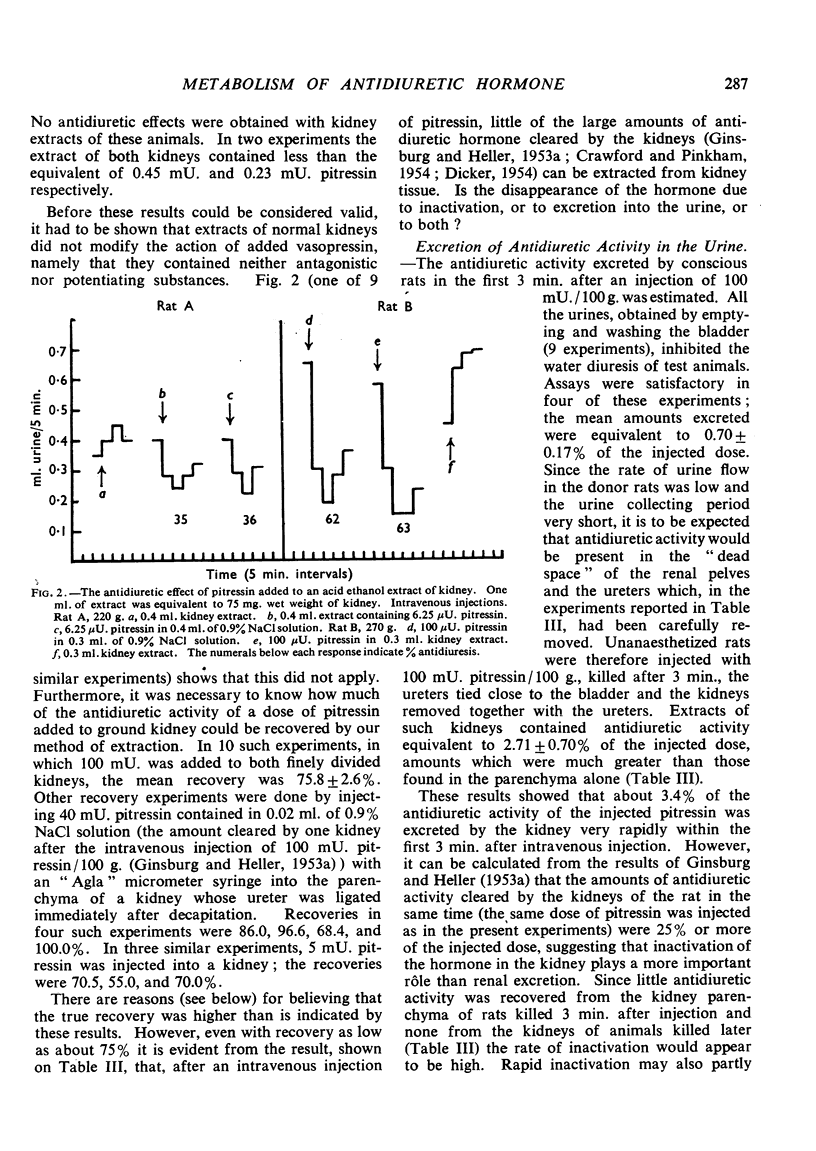
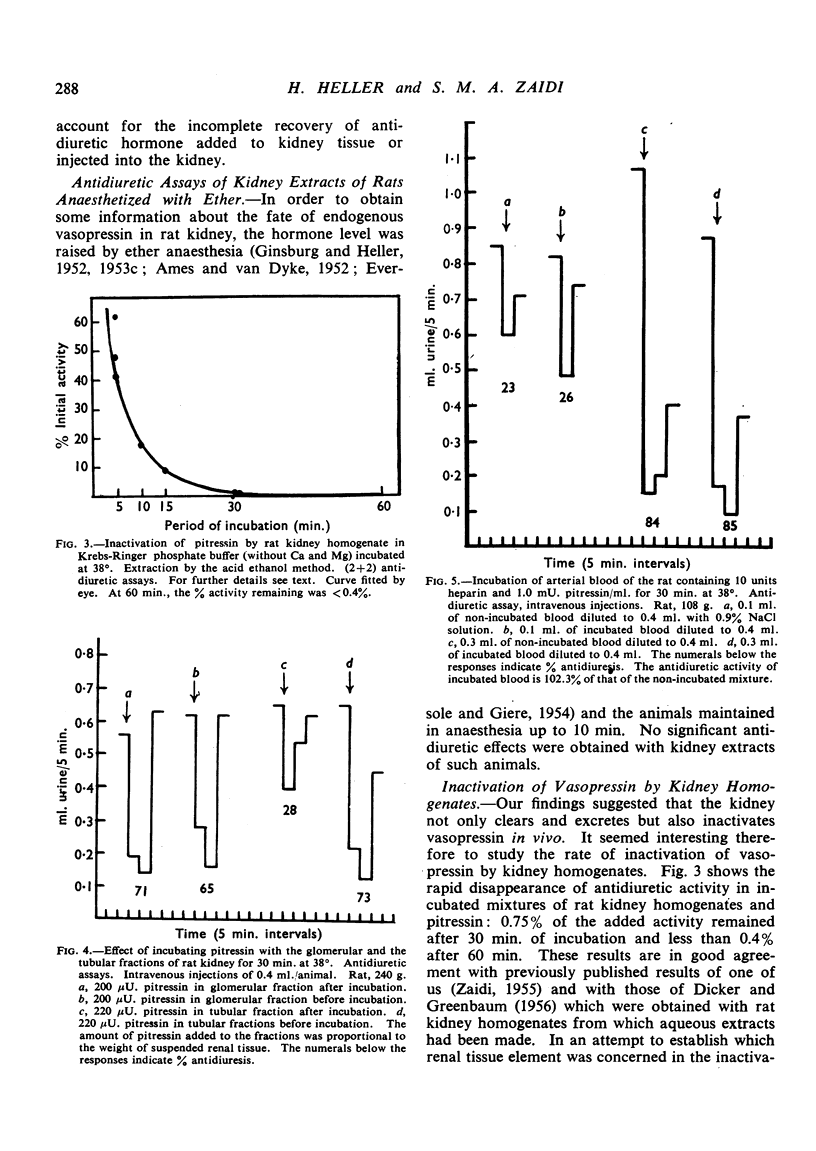
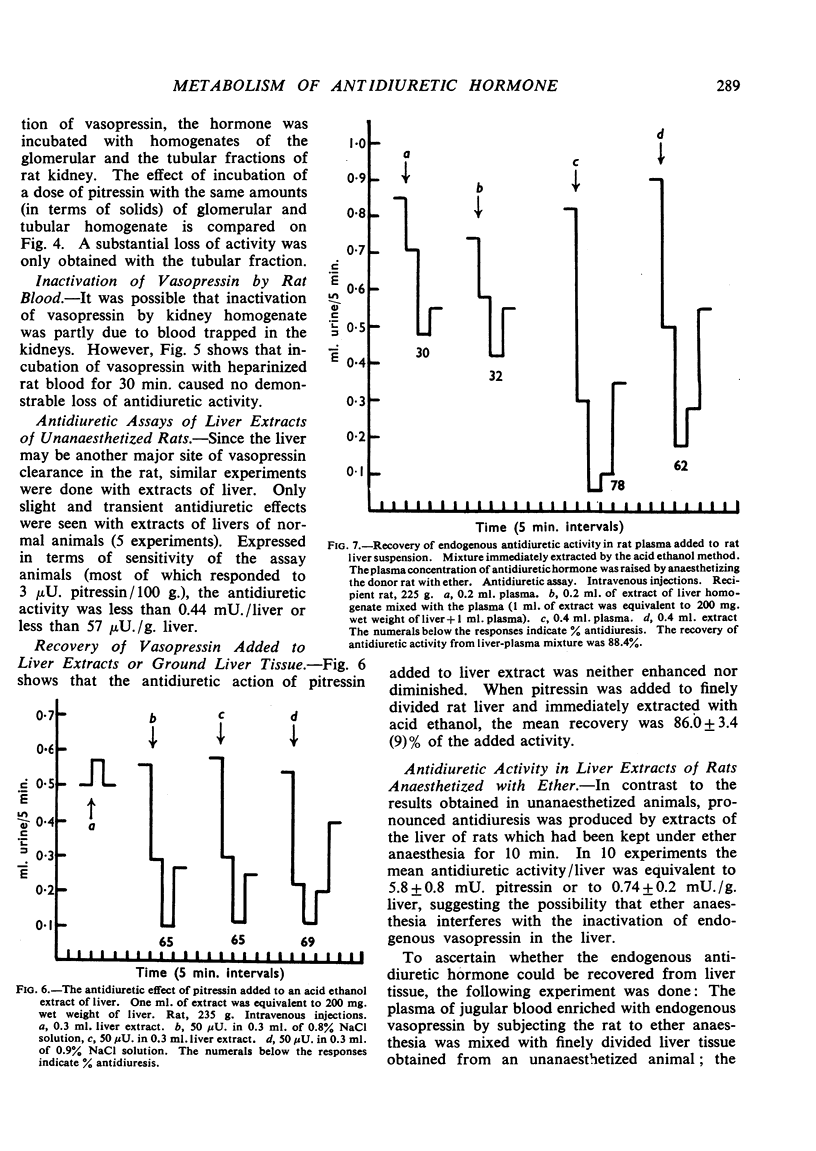
Negligible antidiuretic activity (less than 0.17 mU./g.) was found in extracts of the kidneys either of unanaesthetized adult rats in normal water balance or of rats in whose blood a rise of the level of endogenous antidiuretic hormone had been induced by ether anaesthesia. Extracts of the livers of unanaesthetized rats had negligible antidiuretic activity (less than 0.06 mU./g.), but liver extracts from rats anaesthetized with ether showed antidiuretic effects equivalent to 0.74±0.24 mU. Pitressin/g. liver. When Pitressin was injected intravenously into unanaesthetized rats, small amounts of antidiuretic activity were occasionally found in the livers and the kidneys of animals killed up to 3 min. after the injection but none in animals killed later. Some 3% of the antidiuretic activity of an injected dose of Pitressin was found in the urine and the “dead space” of the kidneys in rats decapitated 3 min. after the intravenous injection. When Pitressin was added to rat kidney homogenate and the mixture was incubated at 38°, only 0.75% of the initial antidiuretic activity was recovered after 30 min. and less than 0.40% after 60 min. Experiments with “glomerular” and “tubular” fractions of rat kidney indicated that the inactivation was essentially due to tubular tissue. It is suggested that, in the rat, the kidneys and perhaps the liver are not only sites of clearance of the antidiuretic hormone but also sites of irreversible inactivation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES R. G., VAN DYKE H. B. Thioglycollate inactivation of posterior pituitary antidiuretic principle as determined in the rat. Proc Soc Exp Biol Med. 1951 Mar;76(3):576–578. doi: 10.3181/00379727-76-18563. [DOI] [PubMed] [Google Scholar]
- BISSET G. W., WALKER J. M. Assay of oxytocin in blood. J Physiol. 1954 Dec 10;126(3):588–595. doi: 10.1113/jphysiol.1954.sp005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD J. D., PINKHAM B. The removal of circulating antidiuretic hormone by the kidney. Endocrinology. 1954 Nov;55(5):699–700. doi: 10.1210/endo-55-5-699. [DOI] [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E. The fate of the antidiuretic activity of pitressin in rats. J Physiol. 1954 Jun 28;124(3):464–475. doi: 10.1113/jphysiol.1954.sp005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELGEE N. J., WILLIAMS R. H. Degradation of insulin-I131 by liver and kidney in vivo. Proc Soc Exp Biol Med. 1954 Nov;87(2):352–355. doi: 10.3181/00379727-87-21380. [DOI] [PubMed] [Google Scholar]
- EVERSOLE W. J., BIRNIE J. H., GAUNT R. Inactivation of posterior pituitary antidiuretic hormone by the liver. Endocrinology. 1949 Oct;45(4):378–382. doi: 10.1210/endo-45-4-378. [DOI] [PubMed] [Google Scholar]
- GINSBURG M., HELLER H., ZAIDI S. M. Re-appraisal of the evidence for the metabolic conversion of vasopressin into a less active derivative. Nature. 1956 Oct 13;178(4537):803–804. doi: 10.1038/178803b0. [DOI] [PubMed] [Google Scholar]
- HAUGAARD N., VAUGHAN M., HAUGAARD E. S., STADIE W. C. Studies of radioactive injected labeled insulin. J Biol Chem. 1954 Jun;208(2):549–563. [PubMed] [Google Scholar]
- Heller H., Urban F. F. The fate of the antidiuretic principle of postpituitary extracts in vivo and in vitro. J Physiol. 1935 Dec 16;85(4):502–518. doi: 10.1113/jphysiol.1935.sp003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. M., Schlapp W. The action and fate of injected posterior pituitary extracts in the decapitated cat. J Physiol. 1936 Jul 21;87(2):144–157. doi: 10.1113/jphysiol.1936.sp003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWELL D. J., GREENSPON S. A., DRAKOWER C. A., BAIN J. A. Metabolic activity of renal cortical tubular epithelial cells. Am J Physiol. 1953 Mar;172(3):709–717. doi: 10.1152/ajplegacy.1953.172.3.709. [DOI] [PubMed] [Google Scholar]
- ZAIDI S. M. Inactivation of vasopressin by the kidney. J Endocrinol. 1955 Mar;12(2):i–i. [PubMed] [Google Scholar]


