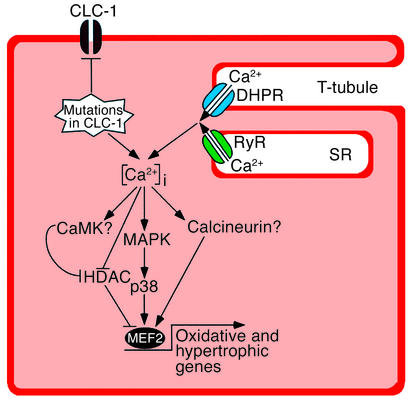
Figure 5.
A model for MEF2 activation in myotonic muscles. CLC-1 chloride channels stabilize membrane potential in normal myofibers. In myotonic myofibers, membrane destabilization causes activation of the stress-responsive p38 MAPK, which is a known activator of MEF2. MEF2 activation in myotonic muscles also correlates with severe deficiency of class II HDACs, which function as repressors of MEF2-dependent gene expression. We propose that activated MEF2 is responsible for altered gene expression profiles in myotonic myofibers (shift toward oxidative phenotype and hypertrophy). Calcineurin and CaMK have previously been shown to activate MEF2. In vitro assays for calcineurin and CaMK showed no change of total enzymatic activities in myotonic muscles (data not shown); however, such assays do not measure potential differences in enzyme activation in vivo. Further in vivo experiments will define the roles of these signaling molecules in regulating gene expression in myotonic muscles. Other calcium-independent signaling pathways might also be responsible for the long-term changes of gene expression patterns in myotonic muscles. DHPR, dihydropyridine receptor; RyR, ryanodine receptor; T-tubule, transverse tubule; SR, sarcoplasmic reticulum.