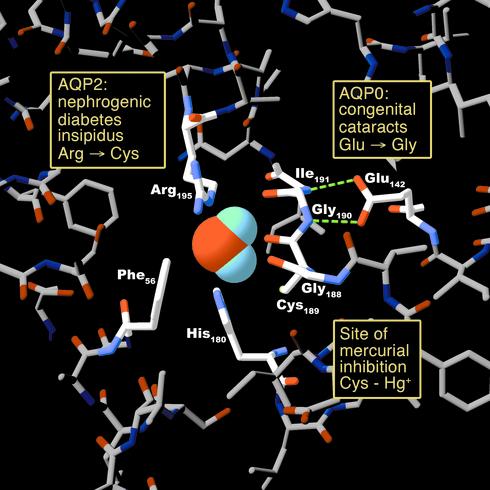
Figure 3.
Human disease mutations and pharmacologically active sites projected onto an atomic model of an AQP1 pore at the narrowest constriction point (transverse section). The model is based on Protein Data Bank coordinates 1H6I of human red cell AQP1 (8). The side chains of three residues (Arg-195, His-180, and Phe-56) plus the backbone carbonyl groups of two residues (Gly-188 and Cys-189) line the pore. Mutation in AQP2 of the residue corresponding to Arg-195 results in autosomal recessive nephrogenic diabetes insipidus. The side chain of Cys-189 lies proximal to the pore. Binding of a mercuric ion to the sulfhydryl group of this residue inhibits the water permeability of AQP1. Hydrogen bonding between Glu-142 and the backbone amide groups of Gly-190 and Ile-191 orients the carbonyl groups of these residues toward the pore. Mutation in AQP0 of the residue corresponding to Glu-142 results in dominantly inherited congenital cataracts.