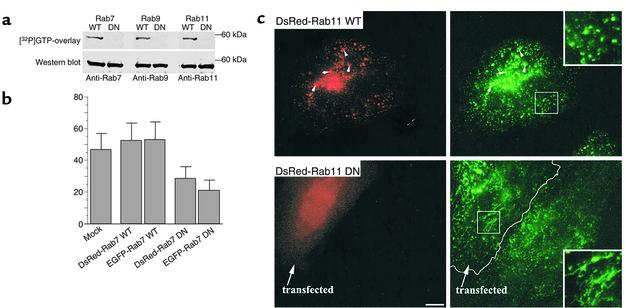
Figure 2.
Characterization of Rab–fluorescent protein constructs. (a) GTP overlay assay and immunoblot analysis of DsRed-Rab fusion proteins. HSFs were transfected with DsRed-Rab7, -Rab9, or -Rab11 (WT or DN) constructs, and 48 hours later cell lysates were prepared. Lysate samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. The blots were incubated for 2 hours with [α-32P]GTP and exposed to x-ray film. The GTP blots were then stripped of GTP and were independently incubated with rabbit anti-Rab7, -Rab9, or -Rab11 monoclonal antibodies. Bands were visualized after secondary antibody incubations by chemiluminescence. (b) Effect of Rab7 WT and mutant protein overexpression on [125I]EGF degradation in HeLa cells. Transfected or mock-transfected cells were incubated with [125I]EGF for 10 minutes at 37°C, washed, and incubated for another 30 minutes at 37°C. TCA-soluble counts in cell lysates and extracellular medium were used to calculate EGF degradation (see Methods). Data are expressed as mean ± SD of three independent experiments. (c) Intracellular localization of Tfn in HeLa cells. Cells overexpressing DsRed-Rab11 WT or DN fusion proteins were incubated with FITC-Tfn for 45 minutes at 37°C. Samples were then washed, acid-stripped to remove fluorescent Tfn from the cell surface, and observed under the fluorescence microscope. In cells overexpressing WT DsRed-Rab11, Tfn colocalized with Rab11 fluorescent protein in punctate vesicular structures, while in cells overexpressing the DN construct, Tfn was found in tubular structures. These distributions are readily seen in the higher-magnification insets (right panels). Bar, 10 μM.