Abstract
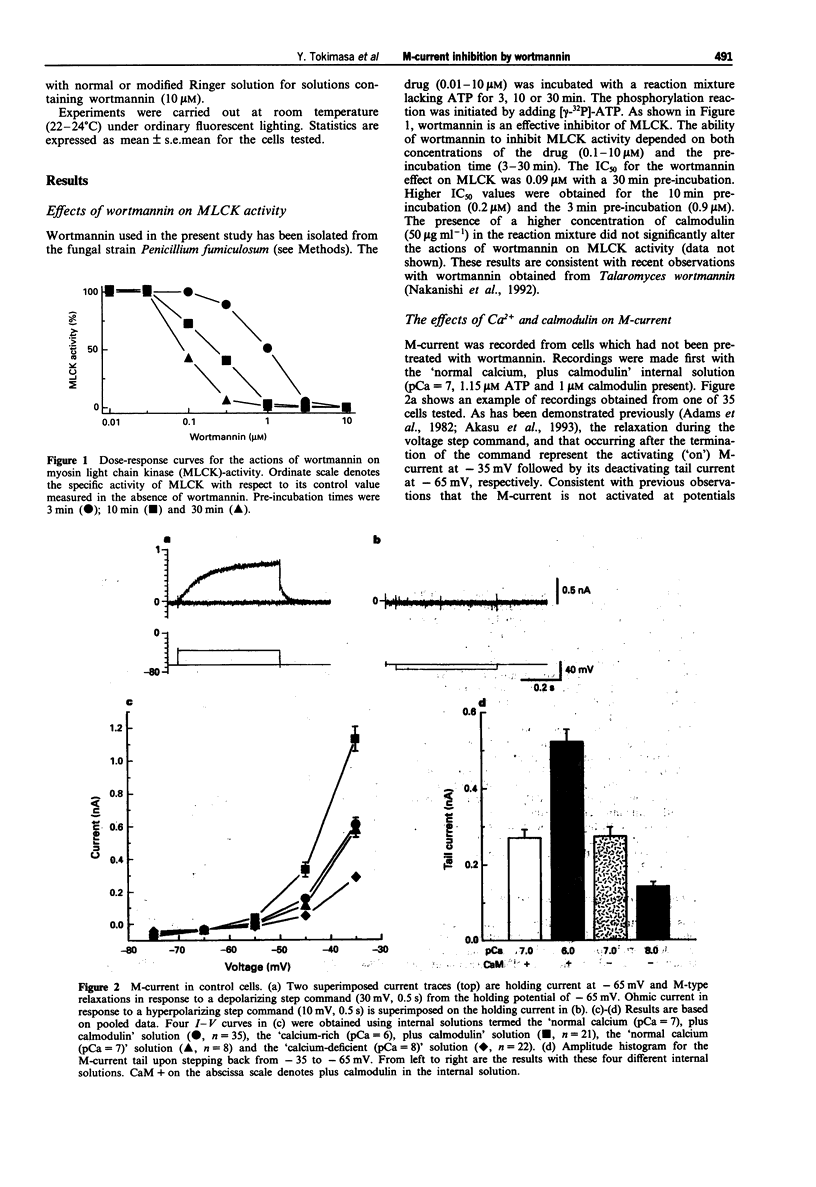
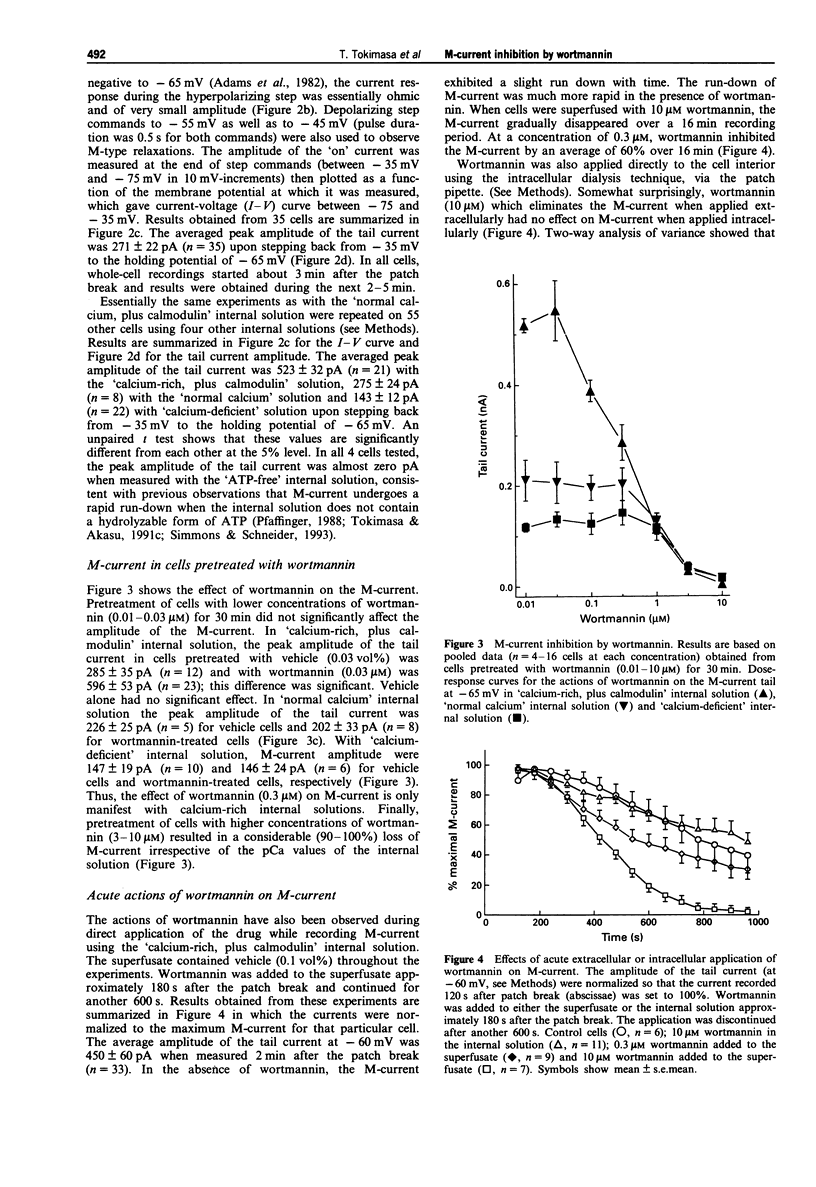
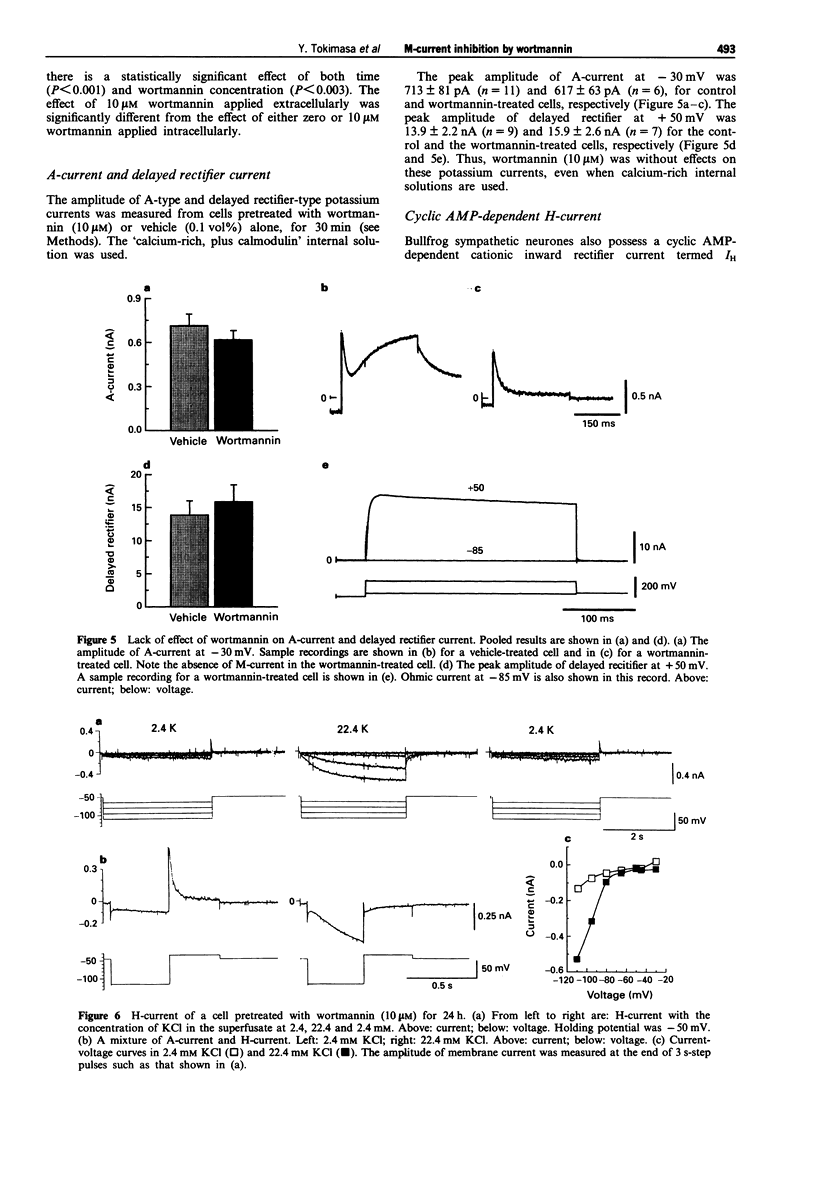
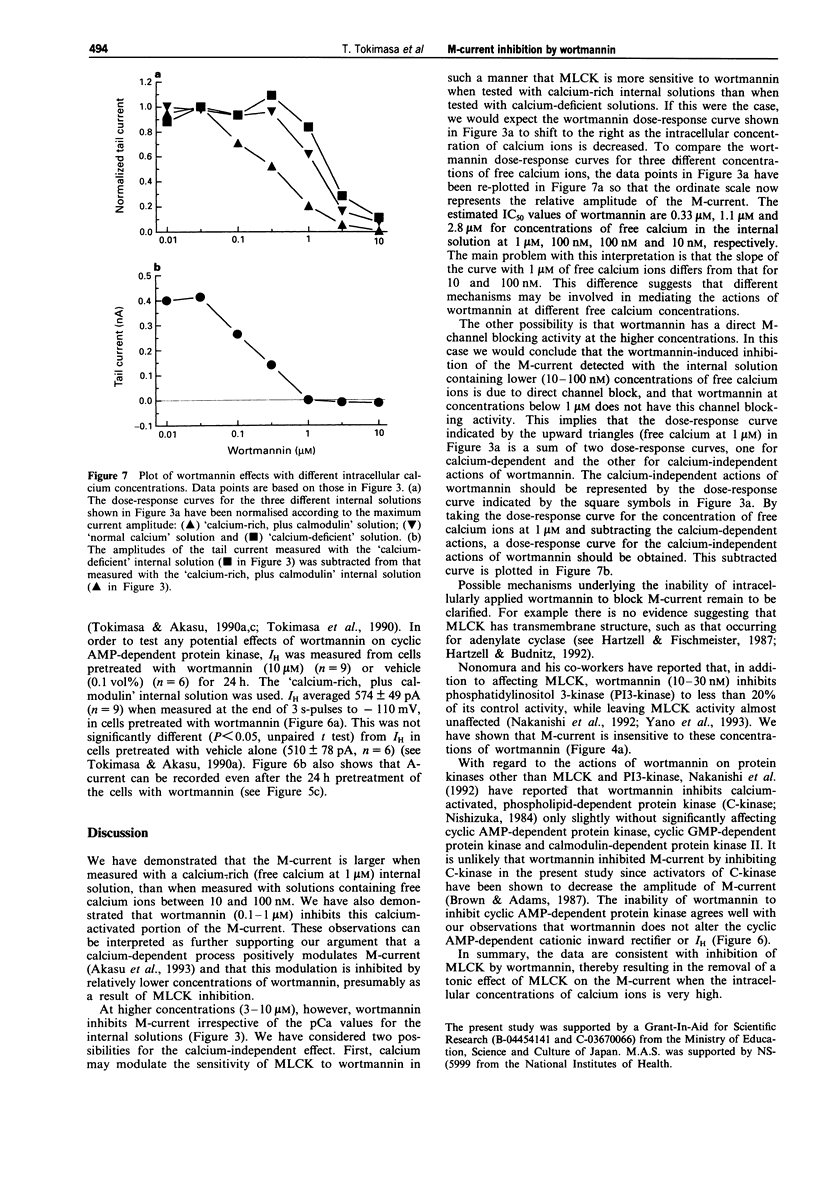
1. The actions of wortmannin, an inhibitor of myosin light chain kinase (MLCK), on M-type potassium current of dissociated bullfrog sympathetic neurones have been examined. 2. The amplitude of M-current was measured by whole cell recordings from cells pretreated with wortmannin (0.01-10 microM) or the wortmannin vehicle, dimethylsulphoxide (0.0001-0.1 vol%), for 30 min. Internal (recording pipette) solutions having three different pCa values (6, 7 and 8) were used for the measurements. 3. Irrespective of the pCa, M-current was not detectable when the cells were pretreated with 10 microM wortmannin. Wortmannin, 3 microM, produced 85-95% inhibition of the M-current. Pretreatment with 10-30 nM wortmannin was without effect on M-current. 4. The M-current inhibition by wortmannin at concentrations of 0.1-1 microM depended on the pCa of the internal solution. Inhibition occurred only when the calcium-rich (pCa = 6) internal solution was used. 5. Pre-treatment of the cells with wortmannin (10 microM) did not affect rapidly-inactivating A-type or delayed rectifier-type potassium currents not did it alter inwardly rectifying sodium-potassium current (IH). 6. These observations show that M-current inhibition by wortmannin has two pharmacological profiles. One is calcium-dependent and occurs at lower concentrations (0.1-1 microM), and is attributed to inhibition of MLCK by wortmannin. At higher concentrations (3-10 microM), wortmannin has an additional, calcium-independent action, inhibiting the M-current by an unknown mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Ito M., Nakano T., Schneider C. R., Simmons M. A., Tanaka T., Tokimasa T., Yoshida M. Myosin light chain kinase occurs in bullfrog sympathetic neurons and may modulate voltage-dependent potassium currents. Neuron. 1993 Dec;11(6):1133–1145. doi: 10.1016/0896-6273(93)90226-h. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Effects of phorbol dibutyrate on M currents and M current inhibition in bullfrog sympathetic neurons. Cell Mol Neurobiol. 1987 Sep;7(3):255–269. doi: 10.1007/BF00711303. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Budnitz D. Differences in effects of forskolin and an analog on calcium currents in cardiac myocytes suggest intra- and extracellular sites of action. Mol Pharmacol. 1992 May;41(5):880–888. [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Effect of forskolin and acetylcholine on calcium current in single isolated cardiac myocytes. Mol Pharmacol. 1987 Nov;32(5):639–645. [PubMed] [Google Scholar]
- Hathaway D. R., Haeberle J. R. Selective purification of the 20,000-Da light chains of smooth muscle myosin. Anal Biochem. 1983 Nov;135(1):37–43. doi: 10.1016/0003-2697(83)90726-1. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Stepinska M., Kemp B. E., Means A. R., Hartshorne D. J. Proteolysis of smooth muscle myosin light chain kinase. Formation of inactive and calmodulin-independent fragments. J Biol Chem. 1987 Oct 5;262(28):13828–13834. [PubMed] [Google Scholar]
- Nakanishi S., Kakita S., Takahashi I., Kawahara K., Tsukuda E., Sano T., Yamada K., Yoshida M., Kase H., Matsuda Y. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J Biol Chem. 1992 Feb 5;267(4):2157–2163. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Olwin B. B., Edelman A. M., Krebs E. G., Storm D. R. Quantitation of energy coupling between Ca2+, calmodulin, skeletal muscle myosin light chain kinase, and kinase substrates. J Biol Chem. 1984 Sep 10;259(17):10949–10955. [PubMed] [Google Scholar]
- Pfaffinger P. Muscarine and t-LHRH suppress M-current by activating an IAP-insensitive G-protein. J Neurosci. 1988 Sep;8(9):3343–3353. doi: 10.1523/JNEUROSCI.08-09-03343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M. A., Becker J. B., Mather R. J. Desensitization of the inhibition of the M-current in sympathetic neurons: effects of ATP analogs, polyanions, and multiple agonist applications. Neuron. 1990 Apr;4(4):557–562. doi: 10.1016/0896-6273(90)90113-t. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. ATP regulates muscarine-sensitive potassium current in dissociated bull-frog primary afferent neurones. J Physiol. 1990 Jul;426:241–264. doi: 10.1113/jphysiol.1990.sp018136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. Cyclic AMP regulates an inward rectifying sodium-potassium current in dissociated bull-frog sympathetic neurones. J Physiol. 1990 Jan;420:409–429. doi: 10.1113/jphysiol.1990.sp017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. Extracellular calcium ions are required for muscarine-sensitive potassium current in bullfrog sympathetic neurons. J Auton Nerv Syst. 1990 Feb;29(2):163–174. doi: 10.1016/0165-1838(90)90182-i. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Sugiyama K., Akasu T., Muteki T. Volatile anaesthetics inhibit a cyclic AMP-dependent sodium-potassium current in cultured sensory neurones of bullfrog. Br J Pharmacol. 1990 Sep;101(1):190–192. doi: 10.1111/j.1476-5381.1990.tb12111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., Tsurusaki M., Akasu T. Slowly inactivating potassium current in cultured bull-frog primary afferent and sympathetic neurones. J Physiol. 1991 Apr;435:585–604. doi: 10.1113/jphysiol.1991.sp018527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., Tsurusaki M., Ishimatsu M., Akasu T. Intracellular ATP changes the voltage-dependence of delayed rectifier potassium current in bullfrog primary afferent neurons. Neurosci Lett. 1993 Dec 12;163(2):138–140. doi: 10.1016/0304-3940(93)90365-r. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Hinkins S., Dabrowska R., Hartshorne D. J. Smooth muscle myosin light chain kinase. Methods Enzymol. 1983;99:279–288. doi: 10.1016/0076-6879(83)99063-8. [DOI] [PubMed] [Google Scholar]
- Yano H., Nakanishi S., Kimura K., Hanai N., Saitoh Y., Fukui Y., Nonomura Y., Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993 Dec 5;268(34):25846–25856. [PubMed] [Google Scholar]
- Yazawa M., Ikura M., Hikichi K., Ying L., Yagi K. Communication between two globular domains of calmodulin in the presence of mastoparan or caldesmon fragment. Ca2+ binding and 1H NMR. J Biol Chem. 1987 Aug 15;262(23):10951–10954. [PubMed] [Google Scholar]


