Abstract
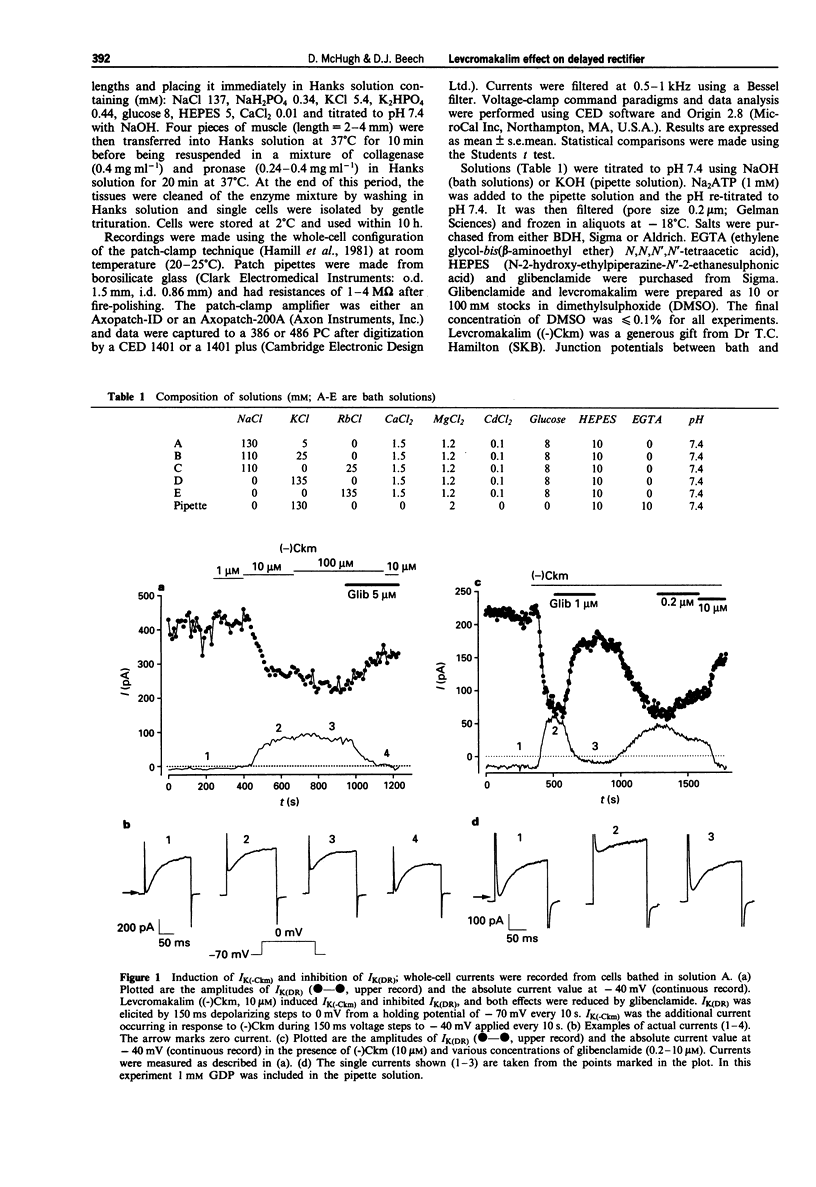
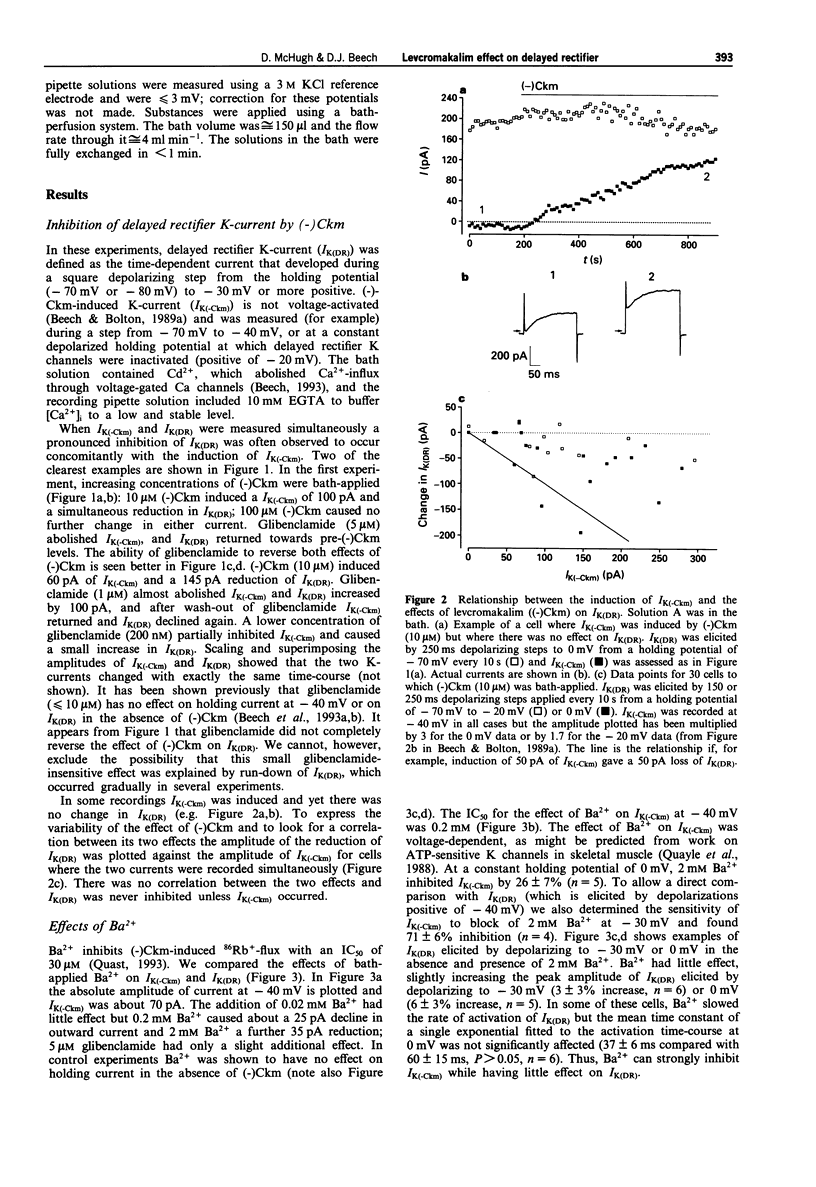
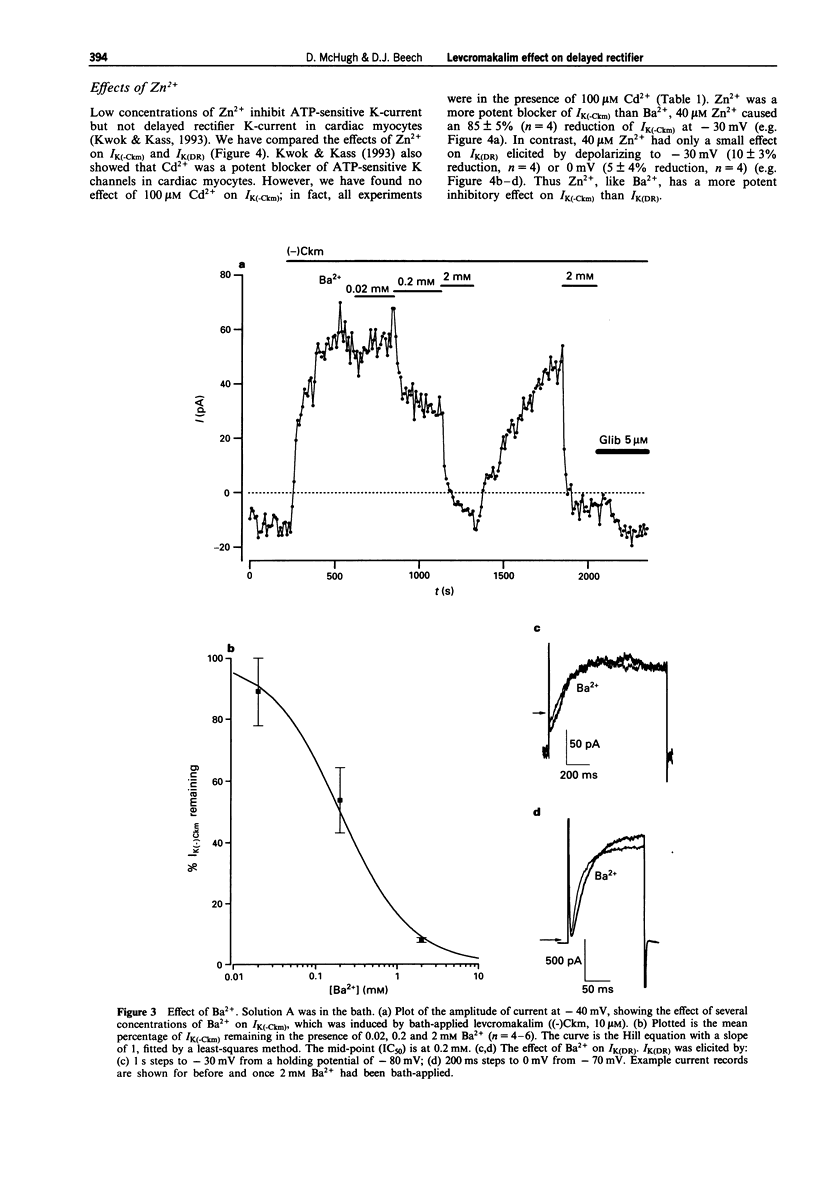
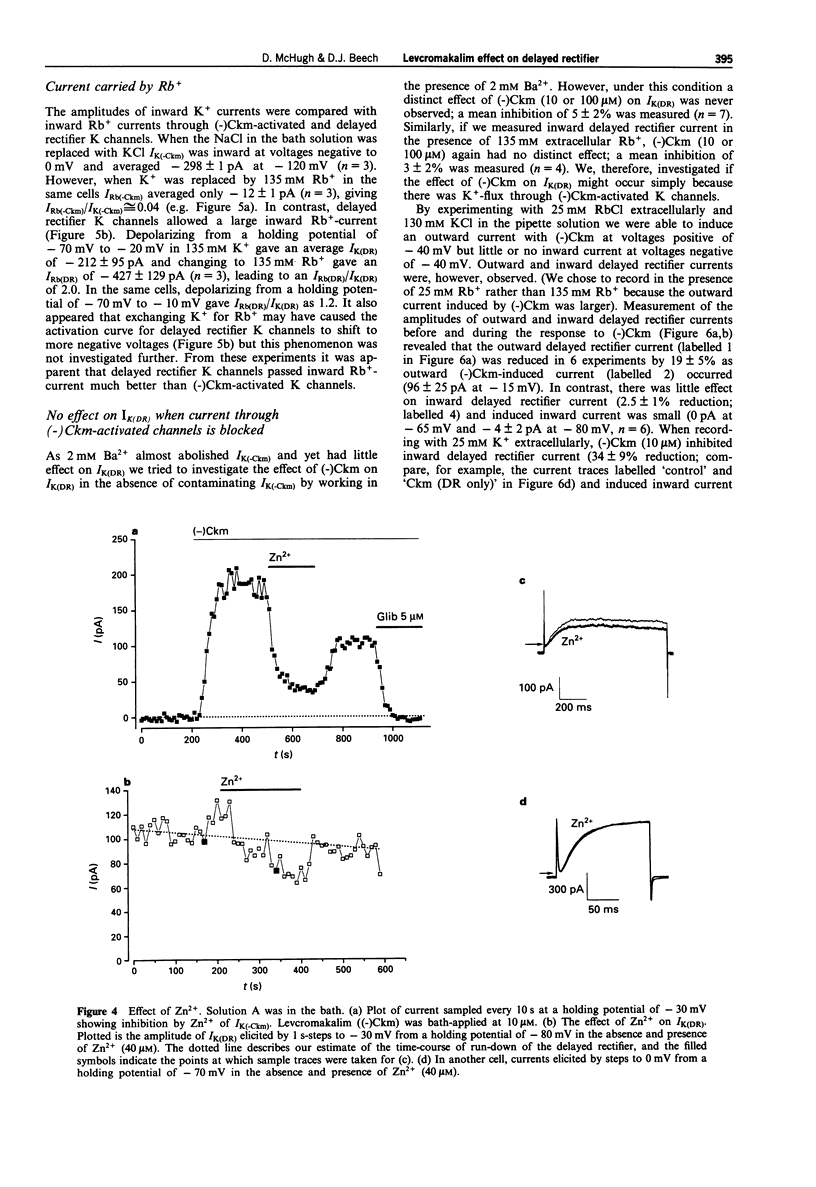
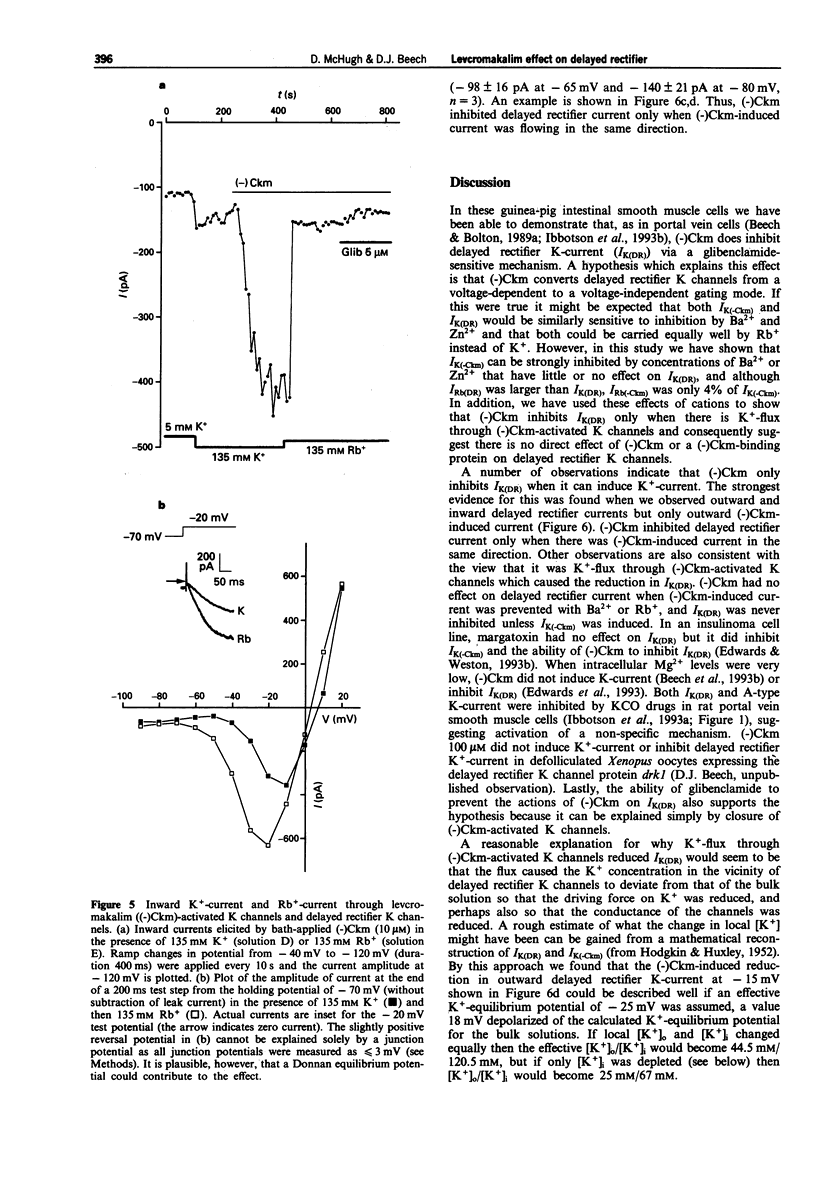
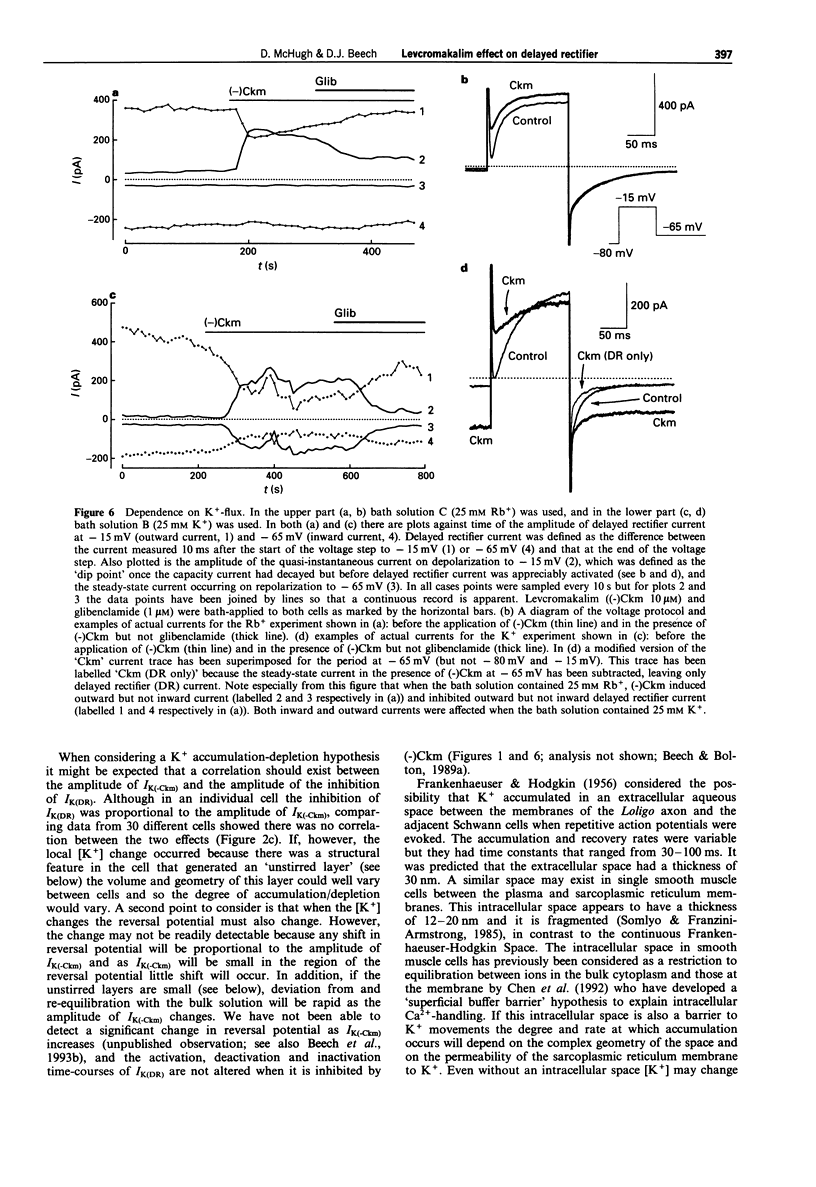
1. Whole-cell voltage-clamp recordings were made from single smooth muscle cells isolated from the longitudinal layer of the guinea-pig small intestine. 2. Levcromakalim ((-)Ckm) inhibited delayed rectifier K-current (IK(DR)) and induced a voltage-independent K-current (IK(-Ckm)). Both effects were inhibited similarly by glibenclamide. In some cells, however, IK(-Ckm) could be induced without any effect on IK(DR). 3. Ba2+ caused a voltage-dependent block of IK(-Ckm). The IC50 was 0.2 mM at -40 mV (6 cells), but at 0 mV 2 mM Ba2+ caused only a 26 +/- 7% inhibition (n = 5). Ba2+ had much less effect on IK(DR), 2 mM Ba2+ having no inhibitory effect on current elicited by depolarization to -30 mV (n = 6) or 0 mV (n = 5). 4. Low concentrations of Zn2+ blocked IK(-Ckm) while having little effect on IK(DR). Zn2+ (40 microM) caused a 77 +/- 1% reduction of IK(-Ckm) at -30 mV (n = 4) but IK(DR) was inhibited by only 10 +/- 3% at the same voltage (n = 4). 5. Inward current amplitudes were compared in 135 mM Rb+ and 135 mM K+ bath solutions. (-)Ckm-activated Rb(+)-current was only 4% of the K(+)-current, whereas delayed rectifier Rb(+)-current was larger than K(+)-current. 6. (-)Ckm did not inhibit IK(DR) if IK(-Ckm) was blocked. In the presence of 2 mM Ba2+ or 135 mM Rb+, (-)Ckm did not induce current nor did it inhibit the delayed rectifier.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Swenson R. P., Jr, Taylor S. R. Block of squid axon K channels by internally and externally applied barium ions. J Gen Physiol. 1982 Nov;80(5):663–682. doi: 10.1085/jgp.80.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford M. L., Bond C. T., Blair T. A., Adelman J. P. Cloning and functional expression of a rat heart KATP channel. Nature. 1994 Aug 11;370(6489):456–459. doi: 10.1038/370456a0. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989 Nov;98(3):851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J. Inhibitory effects of histamine and bradykinin on calcium current in smooth muscle cells isolated from guinea-pig ileum. J Physiol. 1993 Apr;463:565–583. doi: 10.1113/jphysiol.1993.sp019611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Zhang H., Nakao K., Bolton T. B. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993 Oct;110(2):573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Zhang H., Nakao K., Bolton T. B. Single channel and whole-cell K-currents evoked by levcromakalim in smooth muscle cells from the rabbit portal vein. Br J Pharmacol. 1993 Oct;110(2):583–590. doi: 10.1111/j.1476-5381.1993.tb13850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. P., Tomasic M., Kotlikoff M. I. Delayed rectifier potassium channels in canine and porcine airway smooth muscle cells. J Physiol. 1992 Feb;447:329–350. doi: 10.1113/jphysiol.1992.sp019005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Cannell M., van Breemen C. The superficial buffer barrier in vascular smooth muscle. Can J Physiol Pharmacol. 1992 Apr;70(4):509–514. doi: 10.1139/y92-066. [DOI] [PubMed] [Google Scholar]
- Edwards G., Ibbotson T., Weston A. H. Levcromakalim may induce a voltage-independent K-current in rat portal veins by modifying the gating properties of the delayed rectifier. Br J Pharmacol. 1993 Nov;110(3):1037–1048. doi: 10.1111/j.1476-5381.1993.tb13918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Induction of a glibenclamide-sensitive K-current by modification of a delayed rectifier channel in rat portal vein in insulinoma cells. Br J Pharmacol. 1993 Dec;110(4):1280–1281. doi: 10.1111/j.1476-5381.1993.tb13955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Divalent cations and the activation kinetics of potassium channels in squid giant axons. J Gen Physiol. 1982 Jun;79(6):965–996. doi: 10.1085/jgp.79.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogol E., Unwin N. Organization of connexons in isolated rat liver gap junctions. Biophys J. 1988 Jul;54(1):105–112. doi: 10.1016/S0006-3495(88)82935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Weir S. W., Weston A. H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br J Pharmacol. 1986 May;88(1):103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath B. M., Terrar D. A. Effect of glibenclamide, forskolin, and isoprenaline on the parallel activation of KATP and reduction of IK by cromakalim in cardiac myocytes. Cardiovasc Res. 1994 Jun;28(6):818–822. doi: 10.1093/cvr/28.6.818. [DOI] [PubMed] [Google Scholar]
- Ibbotson T., Edwards G., Noack T., Weston A. H. Effects of P1060 and aprikalim on whole-cell currents in rat portal vein; inhibition by glibenclamide and phentolamine. Br J Pharmacol. 1993 Apr;108(4):991–998. doi: 10.1111/j.1476-5381.1993.tb13496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbotson T., Edwards G., Weston A. H. Antagonism of levcromakalim by imidazoline- and guanidine-derivatives in rat portal vein: involvement of the delayed rectifier. Br J Pharmacol. 1993 Dec;110(4):1556–1564. doi: 10.1111/j.1476-5381.1993.tb14001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajioka S., Kitamura K., Kuriyama H. Guanosine diphosphate activates an adenosine 5'-triphosphate-sensitive K+ channel in the rabbit portal vein. J Physiol. 1991 Dec;444:397–418. doi: 10.1113/jphysiol.1991.sp018885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamouchi M., Kajioka S., Sakai T., Kitamura K., Kuriyama H. A target K+ channel for the LP-805-induced hyperpolarization in smooth muscle cells of the rabbit portal vein. Naunyn Schmiedebergs Arch Pharmacol. 1993 Mar;347(3):329–335. doi: 10.1007/BF00167453. [DOI] [PubMed] [Google Scholar]
- Kwok W. M., Kass R. S. Block of cardiac ATP-sensitive K+ channels by external divalent cations is modulated by intracellular ATP. Evidence for allosteric regulation of the channel protein. J Gen Physiol. 1993 Oct;102(4):693–712. doi: 10.1085/jgp.102.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Quast U. Do the K+ channel openers relax smooth muscle by opening K+ channels? Trends Pharmacol Sci. 1993 Sep;14(9):332–337. doi: 10.1016/0165-6147(93)90006-6. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B., Stanfield P. R. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol. 1988 Nov;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. N., Smirnov S. V., Aaronson P. I. Effects of BRL 38227 on potassium currents in smooth muscle cells isolated from rabbit portal vein and human mesenteric artery. Br J Pharmacol. 1992 Mar;105(3):549–556. doi: 10.1111/j.1476-5381.1992.tb09017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Franzini-Armstrong C. New views of smooth muscle structure using freezing, deep-etching and rotary shadowing. Experientia. 1985 Jul 15;41(7):841–856. doi: 10.1007/BF01970000. [DOI] [PubMed] [Google Scholar]
- Volk K. A., Shibata E. F. Single delayed rectifier potassium channels from rabbit coronary artery myocytes. Am J Physiol. 1993 Apr;264(4 Pt 2):H1146–H1153. doi: 10.1152/ajpheart.1993.264.4.H1146. [DOI] [PubMed] [Google Scholar]


